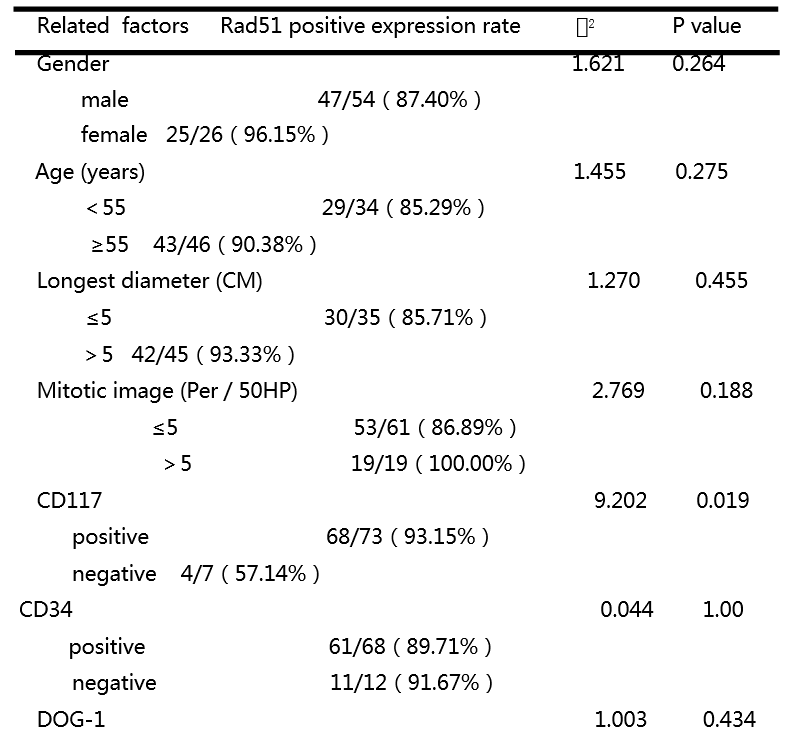
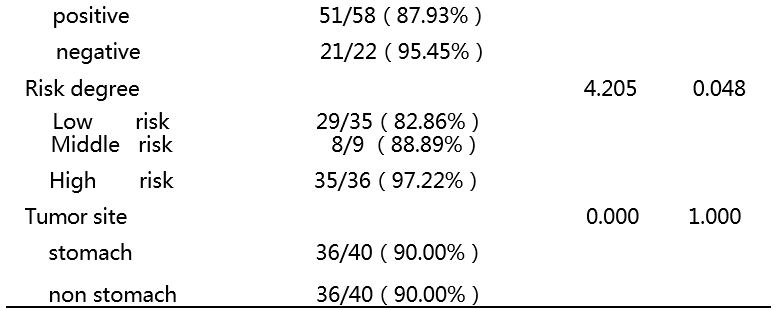
[1] Heinrich M C, Corless C L, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors[J]. Science, 2003, 299(5607): 708-710.
[2] Nakul Valsangkar, MD, Amikar Sehdev, et al. Current management of gastrointestinal stromal tumors:Surgery, current biomarkers, mutations,and therapy [J]. Surgery ,2015,158:1149-64.
[3]Antonescu C R. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial.[J]. Lancet, 2009, 373(9669):1097-1104.
[4] Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastaticgastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625.
[5] ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25(Suppl 3):iii21–6
[6] Zhang Q, Xu J, Qian Y, et al. Association of imatinib plasma concentration and single nucleotide polymorphisms with adverse drug reactions in patients with gastrointestinal stromal tumors. Mol Cancer Ther. 2018;17:2780–2787
[7] Bauer S, Joensuu H, et al. Emerging Agents for the Treatment of Advanced, Imatinib-Resistant Gastrointestinal Stromal Tumors: Current Status and Future Directions. Drugs 2015;75(12):1323-34.
[8] Takahashi T, Elzawahry A, Mimaki S, et al. Genomic and transcriptomic analysis of imatinib resistance in gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2017;56:303–313.
[9] 2017NCCN Clinical Practice Guidelines(V2).
[10]Crosby JA, Catton CN, et al.Malignant gastrointestinal stromal tumors of the small intestine:a review of 50 cases from a prospective database. Ann Surg Oncol.2001;8(1):50–9.
[11]Pierie JP, Choudry U, et al. The effect of and surgery grade on outcome of gastrointestinal stromal tumors.Arch Surg.2001; 136(4):383–9.
[12]Aparicio S, Caldas C. The implications of clonal genome evolution for cancer medicine. N Engl J Med. 2013;368(9):842–851.
[13]Di Scioscio V, Greco L, Pallotti MC, et al. Three cases of bone metastases in patients with gastrointestinal stromal tumors. Rare Tumors.2011;3(2):e17.
[14]Hurwitz J, Constantinidou A, Saran F, et al. The role of radiotherapy in metastatic gastrointestinal stromal tumor (GIST). Proc Connect Tissue Oncol Soc. 2008(2008): abst 35023.
[15]Benjamin D. Goodman DO , Edward M. Mannina MD, et al. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Practical Radiation Oncology (2015) xx, xxx–xxx.
[16]Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases:a subset of metastases curable with stereotactic radiotherapy.J Clin Oncol. 2013;31(11):1384–1390.
[17] Gatto et al. Radiotherapy in the management of gist: state of the art and new potential scenarios.Clin Sarcoma Res (2017) 7:1.
[18] Takeshi Gohongi, Hiroyuki Iida, et al. Postsurgical radiation therapy for gastric carcinosarcoma with c-kit expression: A case report. World J Gastroenterol 2015 ;21(9): 2830-2835.
[19] Joshua Halpern,Yong-June Kim,et al.Effectiveness of radiation therapy in GIST:A case report.Journal of Gastrointestinal Oncology. 2012;2(3):143-146.
[20] Cem Borubana, Orhan Sencanb, et al. Metastatic gastrointestinal stromal tumor with long-term response after treatment with concomitant radiotherapy and imatinib mesylate.Anti-Cancer Drugs. 2007;18:969–972.
[21] Kimberly S Corbin1, et al. Considering the role of radiation therapy for gastrointestinal stromal tumor OncoTargets and Therapy 2014:7 713–718.
[22] Cuaron JJ, Goodman KA, Lee N, Wu AJ. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat Oncol. 2013;8(1):274.
[23] Joensuu H, Eriksson M, Collan J, Balk MH, Leyvraz S, Montemurro M.Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: a prospective study. Radiother Oncol. 2015;116(2):233–8.
[24]Radujkovic, A.Dreger, P.Hegenbart, et al. Imatinib-supplemented myeloablative total-body irradiation/cyclophosphamide conditioning prior to allogeneic stem cell transplantation as consolidation treatment in patients with blast crisis of chronic myeloid leukemia. Eur J Haematol. 2014;6(92): 546-9
[25]Kaygusuz-Atagunduz, I.Toptas, T,et al. Newly diagnosed breast cancer in a patient receiving imatinib mesylate. J Cancer Res Therc. 2014;4(10): 1107-8
[26]Qiao, B.Kerr, M.Groselj, B,et al. Imatinib radiosensitizes bladder cancer by targeting homologous recombination. Cancer Res. 2013;5(73): 1611-20.
[27]De Moura, L. R.Marshall, J. C,et al. The effect of imatinib mesylate on the proliferation, invasive ability, and radiosensitivity of retinoblastoma cell lines. 2013;(27): 92-9.
[28]Podtcheko A, Ohtsuru A, et al. Inhibition of ABL tyrosine kinase potentiates radiation-inducedterminal growth arrest in anaplastic thyroid cancer cells. Radiat Res. 2006;165(1):35–42.
[29]Choudhury A, Zhao H, et al. Targeting homologous recombination using imatinib results in enhanced tumor cell chemosensitivity and radiosensitivity. Mol Cancer Ther.2009;8(1):203–13.
[30]Zhu, J.Zhou, L.Wu, G, et al. A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia. EMBO Mol Med. 2013;3(5): 353-65.
[31]Mukhopadhyay Asima,Drew Yvette,Matheson Elizabeth et al. Evaluating the potential of kinase inhibitors to suppress DNA repair and sensitise ovarian cancer cells to PARP inhibitors.[J] .Biochem. Pharmacol., 2018, undefined: undefined.
[32]Ferguson Peter J,Vincent Mark D,Koropatnick James,Synergistic Antiproliferative Activity of the RAD51 Inhibitor IBR2 with Inhibitors of Receptor Tyrosine Kinases and Microtubule Protein.[J] .J. Pharmacol. Exp. Ther., 2018, 364: 46-54.
[33]Zhu Jiewen,Zhou Longen,Wu Guikai et al. A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia.[J] .EMBO Mol Med, 2013, 5: 353-65.
[34]Tarrade Stephane,Bhardwaj Tanya,Flegal Matthew et al. Histone H2AX Is Involved in FoxO3a-Mediated Transcriptional Responses to Ionizing Radiation to Maintain Genome Stability.[J] .Int J Mol Sci, 2015, 16: 29996-30014.
[35]King Harry O,Brend Tim,Payne Helen L et al. RAD51 Is a Selective DNA Repair Target to Radiosensitize Glioma Stem Cells.[J] .Stem Cell Reports, 2017, 8: 125-139.
[36]Ferguson Peter J,Vincent Mark D,Koropatnick James,Synergistic Antiproliferative Activity of the RAD51 Inhibitor IBR2 with Inhibitors of Receptor Tyrosine Kinases and Microtubule Protein.[J] .J. Pharmacol. Exp. Ther., 2018, 364: 46-54.
[37]Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
[38]Tang Siyuan,Liu Bailong,Liu Min et al. Ionizing radiation-induced growth in soft agar is associated with miR-21 upregulation in wild-type and DNA double strand break repair deficient cells.[J] .DNA Repair (Amst.), 2019, 78: 37-44.
[39]Biermann Jana,Langen Britta,Nemes Szilárd et al. Radiation-induced genomic instability in breast carcinomas of the Swedish haemangioma cohort.[J] .Genes Chromosomes Cancer, 2019, undefined: undefined.
[40]Otsubo Ryota,Matsuda Katsuya,Mussazhanova Zhanna et al. A novel diagnostic method for thyroid follicular tumors based on immunofluorescence analysis of p53-binding protein 1 expression: detection of genomic instability.[J] .Thyroid, 2019, undefined: undefined.