Results
Search results
From the aforementioned search terms, 631 RCTs were initially retrieved. Of these, 415 duplicates were excluded using EndNote X926. Moreover, 199 citations that were noncompliant with the inclusion criteria were excluded after their titles and abstracts were screened. The full texts of the remaining 17 papers was screened, revealing three articles without a full text available, five that did not compare ultrasound- and landmark-guided corticosteroid injection, and one case of CTS not diagnosed through a nerve conduction test. Finally, eight articles were selected for this systematic review and meta-analysis (Fig. 1)27-34.
Study characteristics
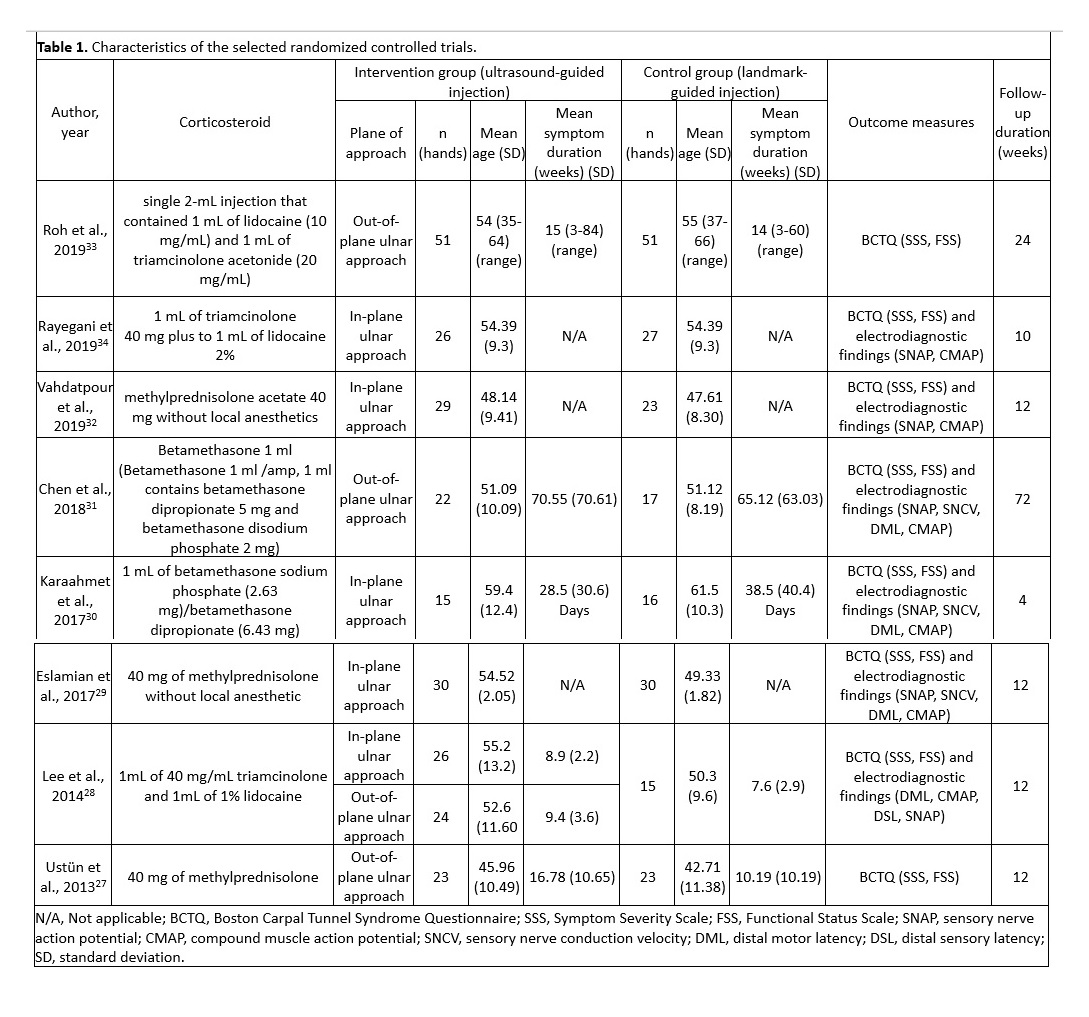
The selected studies were published between 2013 and 2019 and included 448 patients (246 patients in the ultrasound-guided group and 202 in the landmark-guided group). Four studies employed the out-plane ulnar approach27,28,31,33, and five studies adopted the in-plane ulnar approach 28-30,32,34. The main characteristics of the eight RCTs are summarized in Table 1.
Risk‑of‑bias assessment
Two reviewers assessed the quality of the selected RCTs by using the RoB 2 tool, a revision of the Cochrane RoB tool for randomized controlled trials23. Fig. 2 illustrates the risk of bias for each study. Eight studies were identified as having a low risk during randomization27-34. The risk of deviations from intended interventions was low in four studies28,29,31,32, whereas some concerns were noted for the remaining four 27,30,33,34. Eight studies were identified as having a low risk related to missing outcome data27-34. Furthermore, for outcome measures, three studies exhibited uncertain risk 27,33,34, one exhibited high risk30, and four exhibited low risk 28,29,31,32. In terms of the selection of reported results, three studies exhibited low risk29,31,34, but some concerns were noted for the remaining five27,28,30,32,33. The overall risk of bias was low in three studies29,31,32, uncertain in four studies27,28,33,34, and high in one study30.
BCTQ-SSS
BCTQ-SSS scores were reported in all eight studies27-34, including 252 patients in the ultrasound-guided group and 220 in the landmark-guided group. The heterogeneity of the studies was acceptable (I2 = 40%, P = 0.10). The BCTQ-SSS score was significantly lower in the ultrasound-guided group than in the control group [SMD = −0.49, 95% CI (−0.74, −0.25), P < 0.0001]. A subgroup analysis revealed significant differences in BCTQ-SSS score between the ultrasound-guided and control groups for the in-plane ulnar approach [SMD = −0.56, 95% CI (−0.99, −0.14), P = 0.009] and the out-of-plane ulnar approach [SMD = −0.39, 95% CI (−0.65, −0.12), P = 0.004] (Fig. 3).
BCTQ-FSS
BCTQ-FSS scores were reported in all eight studies27-34, which included 252 patients in the ultrasound-guided group and 220 in the landmark-guided group. The homogeneity of the studies was good (I2 = 0%, P = 0.72). BCTQ-FSS scores were significantly lower in the ultrasound-guided group than in the control group [SMD = −0.24, 95% CI (−0.42, −0.06), P = 0.01]. Subgroup analysis revealed a significant difference in BCTQ-FSS score between the ultrasound-guided and control groups for the out-of-plane ulnar approach [SMD = −0.28, 95% CI (−0.55, −0.02), P = 0.04] but not for the in-plane ulnar approach [SMD = −0.20, 95% CI (−0.45, 0.05), P = 0.12] (Fig. 4).
SNAP
SNAP was reported in six studies28-32,34, including 178 patients in the ultrasound-guided group and 146 in the landmark-guided group. The heterogeneity of the studies was moderate (I2 = 62%, P = 0.02). No significant intergroup differences were noted in SNAP [SMD = −0.19, 95% CI (−0.56, 0.17), P = 0.30]. Moreover, subgroup analysis revealed no significant differences in SNAP between the ultrasound-guided and control groups for the in-plane ulnar approach [SMD = −0.27, 95% CI (−0.76, 0.22), P = 0.28] or the out-of-plane ulnar approach [SMD = 0.02, 95% CI (−0.43, 0.47), P = 0.93] (Fig. 5).
SNCV
SNCV was reported in three studies29-31, including 73 patients in the ultrasound-guided group and 66 in the landmark-guided group. The heterogeneity of the studies was moderate (I2 = 53%, P = 0.12). No significant intergroup differences were noted for SNCV [SMD = 0.05, 95% CI (−0.45, 0.55), P = 0.85]. Subgroup analysis revealed no significant differences in SNAP between the ultrasound-guided and control groups for the in-plane ulnar approach [SMD = 0.10, 95% CI (−0.74, 0.94), P = 0.82] or the out-of-plane ulnar approach [SMD = −0.01, 95% CI (−0.64, 0.63), P = 0.98] (Fig. 6).
DML
DML was reported in four studies28-31, including 123 patients in the ultrasound-guided group and 96 in the landmark-guided group. The heterogeneity of the studies was acceptable (I2 = 35%, P = 0.19). DML was significantly lower in the ultrasound-guided group than in the control group [SMD = −0.36, 95% CI (−0.70, −0.02), P = 0.04]. Subgroup analysis revealed a significant difference in DML between the ultrasound-guided and control groups for the in-plane ulnar approach [SMD = −0.51, 95% CI (−0.98, −0.04), P = 0.03] but not for the out-of-plane ulnar approach [SMD = −0.11, 95% CI (−0.57, 0.34), P = 0.63] (Fig. 7).
CMAP
CMAP was reported in six studies28-32,34, including 178 patients in the ultrasound-guided group and 146 in the landmark-guided group. The homogeneity of the studies was high (I2 = 0%, P = 0.82). CMAP was significantly higher in the ultrasound-guided group than in the control group [SMD = 0.38, 95% CI (0.16, 0.61), P = 0.0008]. Subgroup analysis revealed a significant difference in CMAP between the ultrasound-guided and control groups for the in-plane ulnar approach [SMD = 0.42, 95% CI (0.16, 0.67), P = 0.001] but not for the out-of-plane ulnar approach [SMD = 0.27, 95% CI (−0.19, 0.72), P = 0.25] (Fig. 8).