Study design and setting
This retrospective case–control study was conducted at the Gastroenterology Department of the Third Xiangya Hospital of Central South University, Changsha, Hunan Province, People’s Republic of China. We reviewed all the patient records with oesophageal varices who attended our department between February 2015 and February 2020.
Study population
Our study included participants who met the three main inclusion criteria: (1) older than 18 years, (2) having oesophageal varices from any aetiology and (3) underwent emergent or elective BL as a treatment or prophylaxis. These eligible participants were categorized into either the case group or the control group. The case group comprised participants who met the three inclusion criteria and had endoscopically proven bleeding from a BL-induced ulcer (i.e. post-BL ulcer bleeding) without any other cause of bleeding to explain the symptom. In contrast, the control group did not have endoscopically proven bleeding from a BL-induced ulcer. We excluded patients who (1) underwent BL in combination with other haemostasis procedures such as the use of coils or cyanoacrylate injection, (2) died within the first two days following the BL haemostasis procedure, (3) were lost during the follow-up period, (4) had missing data and (5) did not consent to participate in the study.
Band ligation procedure
The Speedband Superview Super 7TM Multiple Band Ligator (Boston Scientific) was used to tie (i.e. strangulate) high-risk varices or actively bleeding varicose veins. Adjuvant pharmacological treatments (i.e. PPIs and antibiotics) and other treatments, such as blood transfusion, hydration, balloon tamponade, transjugular intrahepatic portosystemic shunt and transplantation, were performed according to the hospital’s guidelines [5] at the time and the gastroenterologist’s discretion. All the BL procedures were performed by consultant gastroenterologists, consultant surgeons or specialist trainees under supervision. All the methods were carried out in accordance with relevant guidelines and regulations.
Data collection process
Before collecting the data, we sought approval from the Institutional Review Board of the Third Xiangya Hospital, Central South University, and were assigned approval number 2019-S475.
We utilized the hospital’s electronic patient database to identify eligible patients. The data collected from the eligible patients included demographics, clinical and laboratory parameters and BL procedure outcomes. We independently collected and recorded the data in Microsoft Excel spreadsheets before cross-checking the data for correctness.
Outcomes and variables
The outcome variable was bleeding from a BL-induced ulcer, and the independent variables were obtained from the patients’ demographics, clinical and laboratory parameters and BL procedure outcomes. Further independent variables were identified by reviewing the literature and obtaining experts’ opinions. One BL procedure was considered per participant.
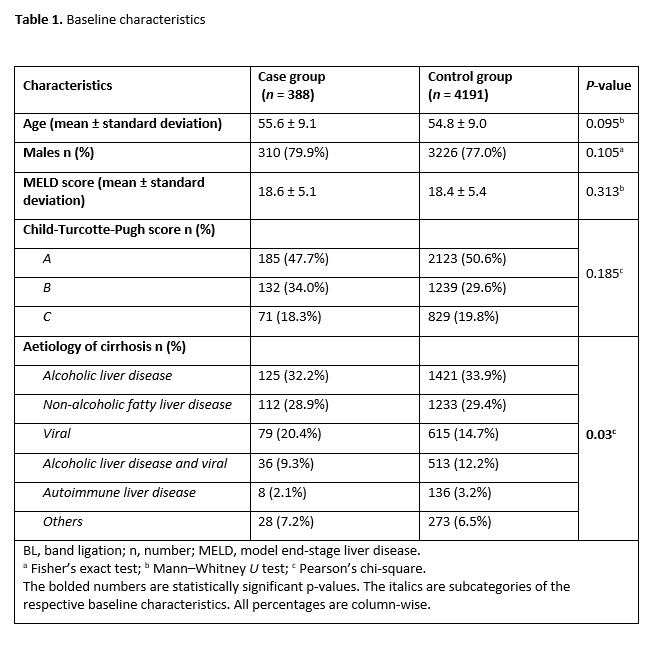
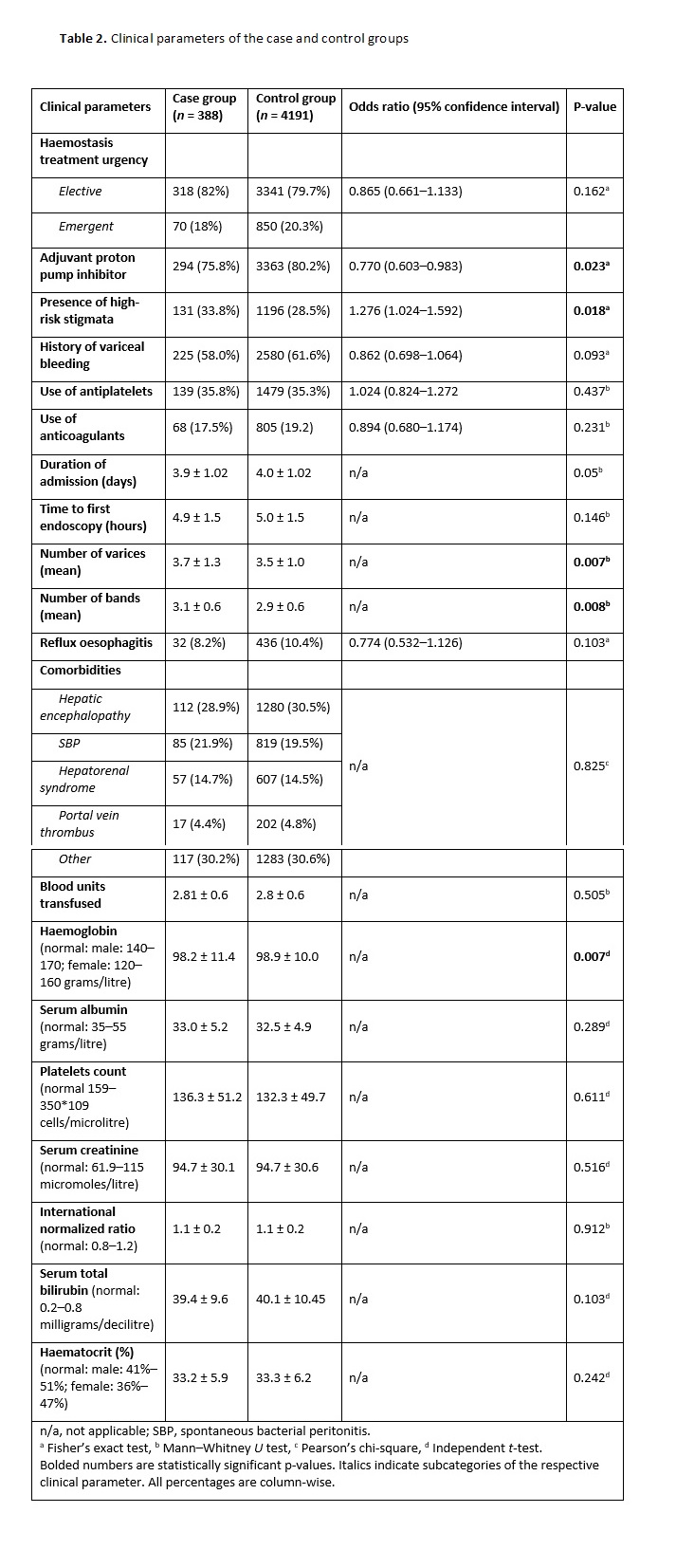
The continuous variables included age, MELD score, duration of admission (in days), time to the first endoscopy (in hours), number of varices, number of bands per variceal site, number of blood units transfused and laboratory investigation results [2, 6]. The categorical variables comprised sex (i.e. male or female), Child-Turcotte-Pugh score (i.e. A, B or C), aetiology of cirrhosis (i.e. alcoholic liver disease, non-alcoholic fatty liver disease, viral, alcoholic liver disease plus viral, autoimmune liver disease or other), haemostasis treatment urgency (i.e. elective or emergent) and adjuvant use of PPIs [12, 13]. The other categorical variables included high-risk stigmata (i.e. yes/no), history of variceal bleeding (i.e. yes/no), use of antiplatelets (i.e. yes/no), use of anticoagulants (i.e. yes/no), reflux oesophagitis (i.e. yes/no) and comorbidities (i.e. hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, portal vein thrombus and others) [10, 14].
Bias mitigation
Both authors independently performed the data collection followed by data cross-checking to ensure the accuracy of the data. Moreover, we utilized the strengthening the reporting of observational studies in epidemiology (STROBE) tool [15] customized for case–control studies in the write-up of this manuscript to reduce reporting bias.
Data analysis
We initially analyzed the patients’ demographic characteristics using mean (and standard deviation) and proportions. For the categorical variables, we used either Pearson’s, chi-square or Fisher’s exact tests to compare the parameters of the case and control groups depending on the respective tests’ assumptions. We performed post-hoc tests by Bonferroni’s adjustment for the crosstabulation of significant categorical variables and calculated the odds ratios. An odds ratio of <1 or >1 corresponded with reduced or increased odds, respectively, of a post-BL bleeding event, while an odds ratio of 1 suggested no association.
For the continuous variables, we utilized either the independent t-test or Mann–Whitney U-test to compare the case and control groups depending on whether the variables demonstrated normal or non-normal distribution curves, respectively. We used the Shapiro–Wilk’s test to determine the statistical significance of normality. A P-value of <0.05 was considered statistically significant.
Additional analysis
We explored the number and causes of death in relation to post-BL ulcer bleeding status. We compared the case and control groups in this respect using Fisher’s exact test and calculated the odds ratio.