Cervical conization is the preferred method for the diagnosis and treatment of CIN. CKC is one type of cervical conization. Because CIN lesions are often multipoint in distribution, residual lesions and positive margins are inevitable. Studies have indicated that the incidence of residual lesions in women with positive margins after cervical conization was higher than that in women with negative margins, and the recurrence rate of women with positive margins was also higher in women with positive margins.[7, 8] It has been reported in the literature that the incidence of positive margins after cervical conization was 14% ~ 25% ,[2, 7, 8] which was similar to our study. However, the rate of residual lesions (33.38%) in our study was higher than in a previous study.[9, 10] This may be due to the clinical characteristics of the population who chose subsequent surgeries. In recent years, the onset age of this disease has been increasingly younger. However, there is no unified clinical opinion on the risk factors and further treatment for the women of child-bearing age with positive margins. In women with no fertility requirements, considering the possibility of a poor prognosis, total hysterectomy may be possible. However, for women of child-bearing age who have fertility requirements or want to retain the uterus, it is almost impossible to accept the uterus being removed. Even secondary conization can affect conception and lead to adverse pregnancy outcomes.[11, 12] Therefore, avoiding a positive margin and residual lesions, and reducing unnecessary secondary surgery are particularly important for women of childbearing age.
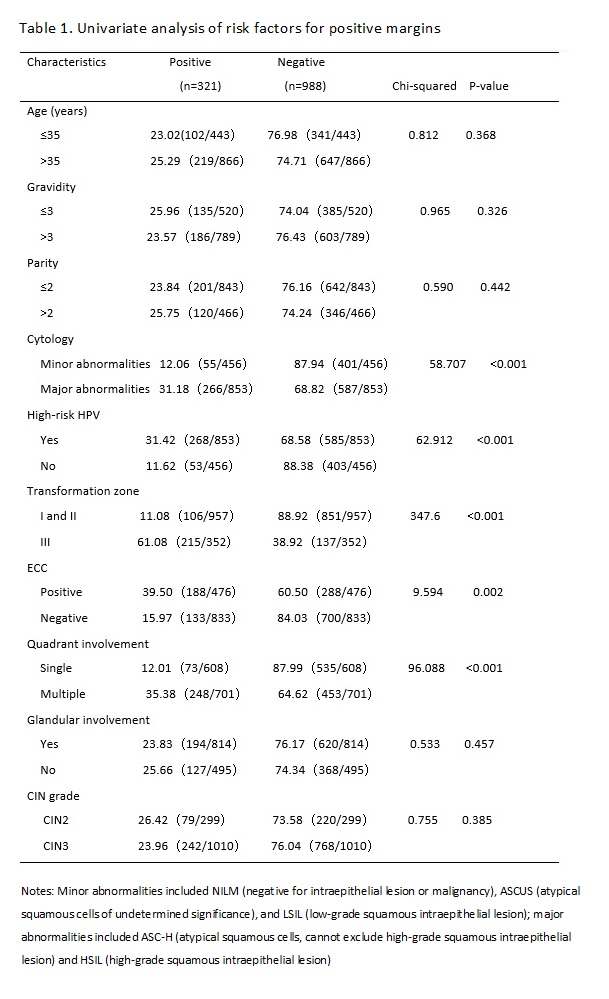
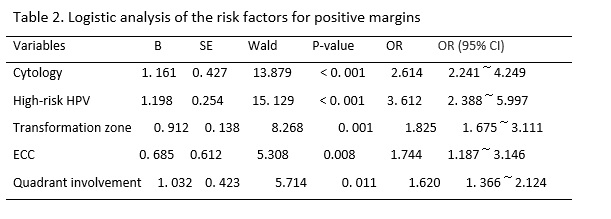
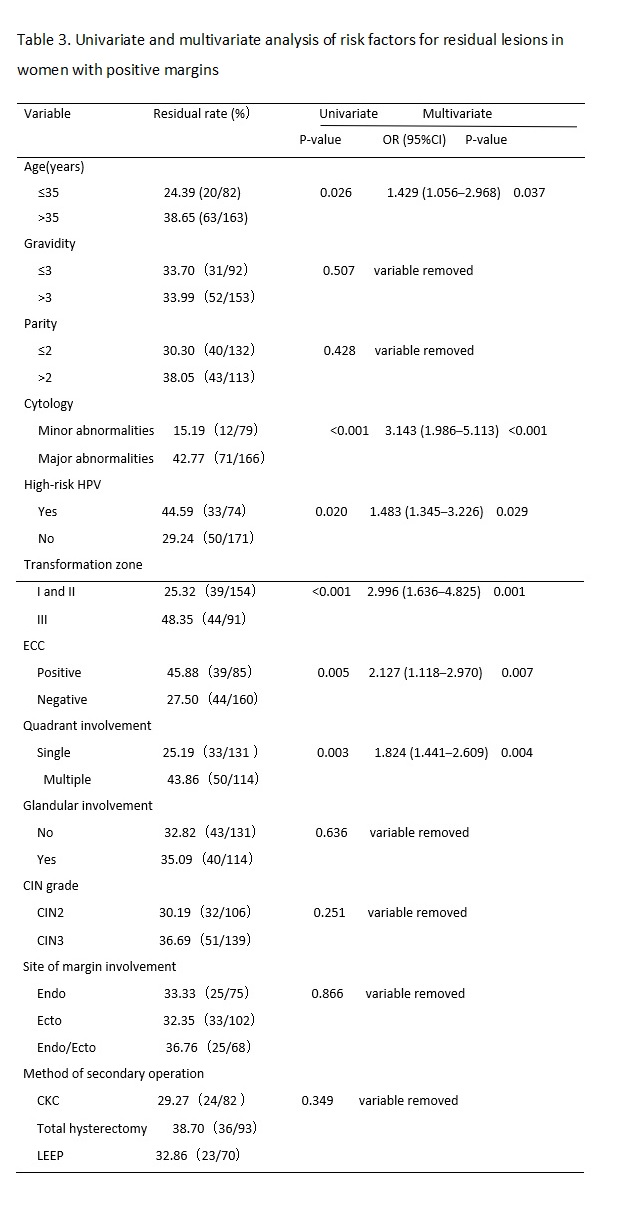
The results of previous studies have suggested that the possible risk factors for residual lesions after cervical conization mainly include positive margins of the specimen, involvement of the lateral margin of the cervical canal, involvement of the lateral margin of the cervical canal and outer orifice, positive specimen from ECC, menopause, persistent infection of high-risk HPV after cervical conization, and decreased or suppressed immune function.[13, 14] Many factors affect the condition of the margin after CKC, including age, menopausal status, glandular involvement, smoking, infection, training level of the operator, and other factors.[15, 16] In this study, we retrospectively analyzed the clinicopathological characteristics of 1,309 women of child-bearing age after initial CKC and 245 cases of women with positive margins who accepted subsequent surgery within three months. We found that a major cytology abnormality (including HSILs and ASC-Hs), high-risk HPV infection type III transformation zone, positive ECC result, and multiple quadrant involvement were the common risk factors for positive margins and residual lesions. Age > 35 years was also a risk factor for residual lesions in women with positive margins after initial CKC.
Whether the severity of a cytological abnormality is related to positive margins has been long disputed by researchers. Ryu A reported that the cytologic grade before cervical conization was not a risk factor for residual disease or recurrence. [17] However, Ayhan showed that the result of a smear was an advantageous predictor for a positive ectocervical margin, and it was associated with a decrease in the occurrence rate of positive margins and residual lesions.[18] In this study, the women with major cytology abnormalities (including ASC-Hs and HSILs) were more likely to present with positive margins and residual lesions than women with minor cytology abnormalities (including NILM, ASCUS, and LSIL) before CKC. We found that the severity of cytology before conization was a risk factor for positive margins and residual lesions. With an increase in the cytological grade, CIN levels increased, which meant that the depth of the lesion cells occupying the squamous epithelium increased, and the possibility of positive margins and residual lesions increased.
High-risk HPV infection has been recognized as a necessary condition for the occurrence and development of cervical squamous epithelial lesions and cervical cancer.[19] In this study, the rate of high-risk HPV infection in women with positive margins after CKC was 83.50% (268/321). Multivariate analysis showed that a high-risk HPV infection was the independent risk factor for positive margins and residual lesions, which was consistent with a previous study.[20, 21] However, at present, the pathological mechanism for a high rate of positive margins and residual lesions after cervical conization caused by high-risk HPV infection is not very clear; it may due to the cervical lesion area in women with CIN infected by high-risk HPV was larger and deeper than that of women infected by low-risk HPV.[22] Further study is needed. Based on the above studies, the HPV type can better predict postoperative positive margins and residual lesions.
In addition, we also found that women with a type III transformation zone were more likely to have positive margins and residual lesions after an operation. This may be due to the lesion invading the cervical tube. CKC cannot completely remove diseased tissue. In the same way, the rates for positive margins and residual lesions in women with positive results of ECC were higher than that of negative results. The same results were found in previous studies.[23, 24] Researchers also found that multiple quadrant involvement was a risk factor for positive margins and residual lesions.[25, 26] We came to the same conclusion based on our study results. In this study, the rate of multiple quadrant involvement was 77.26% in the women with positive margins, and that in the women with negative margins was 45.85%. Increases in the range of lesions likely affected observations during the operation, interfered with the judgment of surgical margins, and increased the difficulty of surgery. The above factors should be taken into account in the process of cervical conization to achieve the goal of leaving no residual lesions and preserving cervical function to a greater extent; that is, we should pay more attention to the extent and depth of lesions removed by cervical conization.
There was no significant difference between women of age > 35 years and ≤ 35 years in the rate of positive margins, but there was a significant difference in the rate of residual lesions between the two age groups. The rate of residual lesions in the age > 35 years group was higher than the ≤ 35 years group (P < 0.03). This may be due to the persistent infection of HPV, especially high-risk HPV. Sarian reported that women older than 35 years had a significantly higher risk for persistent infection following LEEP.[27] This could cause multiple quadrant lesions of the cervix.(30) Furthermore, the older the patient, the higher the degree of cervical atrophy. The cervical transformation zone and lesions therefore moved inward to the cervical canal, so cervical conization could not completely remove the diseased tissue. Bilibio also considered that increasing age was the only factor that accurately predicted residual disease.(18) All of these results indicated older age was a predictive factor for residual lesions and it can play a better role in guiding the formulation of a postoperative treatment plan for women of child-bearing age with positive margins. Subsequently, we can preserve normal reproductive function and organ integrity as much as possible in women younger than 35 years old.