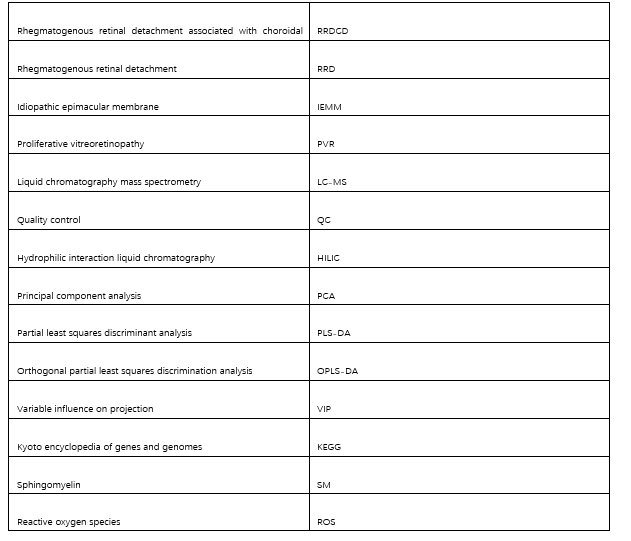
Abbreviations
DOI: https://doi.org/10.21203/rs.3.rs-2660602/v1
Rhegmatogenous retinal detachment associated with choroidal detachment (RRDCD) is a complex type of retinal detachment. This disease is characterized by high difficulty of operation and poor prognosis. However, the pathogenesis is still unclear. The purpose of this study was to analyze the changes of metabolites and metabolic pathways in vitreous fluid of RRDCD patients.
Using ultra-high-performance liquid chromatography coupled with the quadrupole time-of-flight mass spectrometry method, metabolites in the vitreous fluid of RRDCD and idiopathic epimacular membrane (IEMM) patients were analyzed. A total of 28 samples were analyzed to compare the significance of different metabolites between these groups.
We identified 135 different metabolites between the RRDCD and IEMM groups (VIP > 1, P value < 0.05). Compared with the IEMM group, the level of sphingomyelin, adenosine, L-palmitoylcarnitine and 4,7,10,13,1 6,19-docosahexaenoic acid up-regulated in RRDCD group. A series of lipid metabolites were up-regulated in RRDCD group. The main metabolic pathways involved were protein digestion and absorption, ABC transporters, aminoacyl-tRNA biosynthesis, central carbon metabolism in cancer and sphingolipids metabolism.
Our results suggest that the up-regulation of sphingomyelin and abnormal sphingolipids metabolism may induce cell migration and death after retinal or choroidal detachment. And then, it may induce intraocular inflammation and damage visual function. The accumulated L-palmitoylcarnitine and fatty acid metabolites may damage the energy pathway of retina, and aggravate the death of photoreceptors after hypoxia. These results provide clues for studying the mechanism, treatment and prognosis of RRDCD.
Rhegmatogenous retinal detachment associated with choroidal detachment (RRDCD) is a special type of rhegmatogenous retinal detachment (RRD). RRDCD has clinical characteristics of severe vision loss, retinal and choroidal detachment, ocular hypotony, uveitis, and deep anterior chamber 1, 2. The incidence of RRDCD in primary RRD is reported to be 2.0–8.6% 3. However, in China, it is as high as 1.5–18.1%, much higher than the incidence rate of 2.0–4.5% in Western countries 4, 5. RRDCD progresses rapidly, and the prognosis for vision is poor. The probability of proliferative vitreoretinopathy (PVR) after surgery for RRDCD is 35.4–52.4% 3, 6, and the success rate of surgery is usually lower than that of primary RRD5.
The pathogenesis of RRDCD is not clear, but it is currently believed that hypotony will cause choroidal vascular leakage, and the resultant inflammation will lead to suprachoroidal effusion and, eventually, choroidal detachment 1, 7. Choroidal detachment disrupts the aqueous circulation, further reduces intraocular pressure, aggravates the detachment of the retina and choroid; these changes form a vicious cycle resulting in significant morbidity 8. Some studies have shown that oral steroids before vitrectomy can improve the rate of retinal reattachment in patients with RRDCD 9 and reduce postoperative inflammation and PVR incidence 6. This demonstrates that inflammation in RRDCD patients is associated with prognosis. Our previous study also confirmed that inflammatory mediators in the vitreous fluid of RRDCD patients are up-regulated 10. Proteomics results show that the pathogenesis of RRDCD involves the complement pathway and the coagulation cascade pathway 11.
Metabolomics can detect changes in small molecular metabolites of biological systems and help identify potential biomarkers for disease diagnosis and treatment. In this study, we used new metabolomics techniques to analysis vitreous fluid of patients with RRDCD. Liquid chromatography mass spectrometry (LC-MS) is increasingly used in non-targeted metabolomics research due to the simple process of sample processing, high sensitivity, and high accuracy. And we added idiopathic macular epiretinal membrane (IEMM) group as the control group. Intraocular inflammation in IEMM patients was relatively static. This is the first time to compare the vitreous fluid of RRDCD patients with relatively normal vitreous fluid.
In this study, we found significant changes of 135 metabolites levels in RRDCD compared to IEMM groups, which contained L-palmitoylcarnitine, sphingomyelin(d18:1/18:0), indolelactic acid, and 4,7,10,13,16,19-docosahexaenoic acid. Many lipid metabolites were also up-regulated in RRDCD group. These metabolites mainly involved metabolic pathways such as protein digestion and absorption, ABC transporters, aminoacyl-tRNA biosynthesis, central carbon metabolism in cancer and sphingolipids metabolism.
We hope that this study will help to explore the mechanism of RRDCD, improve clinical treatment options, and ultimately improve the prognosis of patients.
We recruited 28 consecutive cases of vitrectomy in Wuxi Second Hospital Affiliated to Nanjing Medical University from March 2019 to January 2020: 16 patients with RRDCD and 12 patients with IEMM. The inclusion criteria were patients with RRDCD or IEMM diagnosed with a slit lamp, optical coherence tomography and ultrasound biomicroscopy before surgery. All patients had no other ocular conditions. The exclusion criteria were (1)patients with other eye diseases, such as eye trauma, glaucoma, retinitis pigmentosa, anterior segment dysgenesis diabetic or hypertensive retinopathy and age-related macular degeneration. ༈2༉PVR caused by other diseases is also excluded and (3) Exclude patients with previous eye surgery history. All patients did not use steroids before surgery. All patients had provided informed consent before being included in the examination. All subjects were subjected to a comprehensive eye examination by an experienced ophthalmologist before surgery. The PVR classification was performed according to the Terminology Committee of the Society of Retina 12.
When the diagnosis was confirmed, a vitrectomy was performed as soon as convenient. To avoid the perfusion fluid influencing vitreous fluid, we strictly followed the surgical method of phacoemulsification and cataract extraction after pars plana vitrectomy.
The patient underwent a 25G vitrectomy under local or general anesthesia. An undiluted vitreous sample (1-2ml) was collected before opening the perfusion system during the operation. The sample was immediately transferred to a centrifuge tube and placed on ice and processed within one hour of collection. Each sample was centrifuged for 10 min at 4°C, 4000r/min, 10 min and the supernatant was collected and frozen at -80°C.
After taking out the samples at -80°C, slowly dissolve them at 4°C. Take 100ul of each group of samples and add 400ul of precooled methanol acetonitrile solution (1:1, v/v). After vortex 60s, place the samples for 1h at -20°C to precipitate the protein. Then these samples were centrifuged for 20 min at 4°C, 14000rcf. Take the supernatant and saved at -80°C.
The vitreous fluid samples were analyzed on an AB Triple TOF 5600/6600 mass spectrometer (AB SCIEX, Framingham, MA, USA) and an Agilent 1290 Infinity LC ultra-high pressure liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA).
Hydrophilic interaction liquid chromatography (HILIC) separation was performed on an ACQUITY UHPLC BEH Amid 1.7µm column (2.1mmx100mm; Waters). The column temperature was maintained at 25°C, and the flow rate was 0.3mL/min. The mobile phase contained 25 mM ammonium acetate and 25 mM ammonium hydroxide in water (A) and acetonitrile (B) in both positive and negative ESI modes. The gradient was 85% B for 1 min and was linearly reduced to 65% over 11 min, then was reduced to 40% in 0.1 min and kept for 4 min, and then increased to 85% in 0.1 min, with a 5 min re-equilibration period employed.
After the samples were separated, they were analyzed using the AB Triple TOF 5600/6600 mass spectrometer in the positive and negative ESI modes. The ESI source conditions were set as follows: Ion Source Gas1 as 60, Ion Source Gas2 as 60, curtain gas as 30, source temperature: 600°C, IonSpray Voltage Floating ± 5500 V. The product ion scan is acquired using information-dependent acquisition with a high sensitivity mode selected. The collision energy was fixed at 35 V with ± 15 eV. The declustering potential was set to ± 60 V.
To evaluate the stability of the instrument performance during the LC-MS analysis, equal amounts of all samples’ extracts were mixed under cold conditions to obtain four quality control (QC) samples. QC samples were randomly inserted into the processing queue to monitor and evaluate the stability of the system and the reliability of the experimental data.
We converted the raw UHPLC-Q-TOF/MS data into mzXML files using ProteoWizard MSConvert and then used XCMS for processing. After Pareto-scaling preprocessed the data, multivariate statistical analysis was performed by using the SIMCA-P software (version 14.1, Umetrics, Umea, Sweden), including principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA) and orthogonal partial least squares discrimination analysis (OPLS-DA). The significant different metabolites were determined based on the combination of a statistically significant threshold of variable influence on projection (VIP) values obtained from PLS-DA model and two-tailed Student’s t test (p value) on the raw data. The metabolites with VIP values larger than 1.0 and p values less than 0. 05 were considered as significant. Pearson correlation analysis was used for processing the data of QC samples. The MetaboAnalyst tool were used for the functional enrichment analysis of the discriminating metabolites. The significant remaining metabolites were identified by KEGG database 33 searches.
Data are expressed as n%. Fold change analysis and student’s t-test were applied for comparison between the RRDCD, RRD and IEMM groups. Chi-square test and one-way analysis of variance were used to analyze the clinical information of these two groups. Differences are considered as statistically significant at p < 0.05. Pearson correlation analysis was used to analyze the results of QC samples.
16 patients with RRDCD and 12 patients with IEMM were recruited in this study. The clinical parameters of the patients are shown in Table.1. There is no statistical significance in terms of sex and age between the two groups (P > 0.05). The average intraocular pressure of the RRDCD group was significantly lower (P < 0.001, P < 0.05).
Table.1. Clinical Characteristics of the study population
|
Clinical Characteristics |
RRDCD, n = 16 |
IEMM, n = 12 |
P |
|---|---|---|---|
|
Sex, n% |
|||
|
Male |
11 (68.8) |
5 (41.7) |
P = 0.152 |
|
Female |
5 (31.2) |
7 (58.3) |
|
|
Age, y |
|||
|
Median(range) |
61.7(40–74) |
64.0(32–81) |
P = 0.605 |
|
PVR grade, n% |
|||
|
Mild, A, B |
14 (87.5) |
- |
|
|
Heavy, C, D |
2 (12.5) |
- |
|
|
IOP |
|||
|
Median(range) |
8.2 |
14.6 |
P < 0.001 |
We tested the 28 vitreous fluid samples. After matching with the database, we eventually identified 250 metabolites in the positive ion mode and 252 metabolites in the negative ion mode. In order to screen differential metabolites from massive metabolomic data and establish an accurate discrimination model, we used partial least squares discrimination analysis (PLS-DA) to analyze data of these two groups (Fig. 1A, B). OPLS-DA score plots show the difference of metabolomics between the two groups (Fig. 1C, D), and Fig. 1E, F show the effectiveness and stability. VIP > 1, P < 0.05 were used as screening criteria for metabolite with significant difference.
Combining the results of POS mode and NEG mode, we found that compared with the IEMM group, there were 135 significantly differential metabolites in RRDCD group. 125 metabolites level up-regulated including L-palmitoylcarnitine, sphingomyelin (d18:1/18:0), indolelactic acid, and 4,7,10,13,16,19-docosahexaenoic acid. 10 metabolites level down-regulated, such as L-gulonicgamma-lactone, L-ascorbic acid and Ile-val (Additional file 1). 16 metabolites were repetitive in both modes. Volcano plots reflected the potential change of metabolites between two groups (Fig. 2). Hierarchical clustering analysis was performed to show the clear distinction in these two groups (Fig. 3).
Kyoto encyclopedia of genes and genomes (KEGG) pathway annotation analysis was performed on the two groups. Compared with IEMM group, significant changes had taken place in such important pathways in RRDCD groups as protein digestion and absorption, ABC transporters, aminoacyl-tRNA biosynthesis, central carbon metabolism in cancer and sphingolipids metabolism (Fig. 4).
RRDCD is a clinically intractable ophthalmic disease. The patients with RRDCD have high surgical difficulty and poor prognosis. However, there are few studies on this disease. In our study, the IOP of RRDCD group was significantly lower than that of IEMM group, and RRDCD patients had different degrees of PVR. Investigating potential sensitive molecular events in vitreous fluid of RRDCD patients can provide clues for exploring the pathogenesis of RRDCD.
Metabolomics has been widely used in screening clinical biomarkers. Compared with the IEMM group, we found that the levels of 125 metabolites in vitreous fluid of RRDCD patients were up-regulated, and the levels of 10 metabolites were down-regulated. L-Palmitoylcarnitine, N-Docosanoyl-4-sphingenyl-1-O-phosphorylcholine, adenosine, sphingomyelin and 4,7,10,13,16,19-docosahexaenoic acid may be potential biomarkers. Protein digestion and absorption, ABC transporters, aminoacyl-tRNA biosynthesis, sphingolipids metabolism and other pathways were significantly interfered in RRDCD group. Revealing the changes of metabolites in vitreous fluid of RRDCD patients can help us discover the pathogenesis of RRDCD, find specific biological targets, and formulate treatment strategies.
Sphingomyelin (SM) is the main component of cell membrane. It has been proved that RPE cells, glial cells and inflammatory cells migrate to vitreous cavity after retinal detachment 13. SM in vitreous fluid of RRDCD patients may come from migrating retinal cells. Several studies have shown that the SM-rich microdomains on the cell membrane act as receptors to regulate a variety of cellular processes such as cell death, proliferation 14, 15 and migration 16. The up-regulated SM level will induce more cell migration and cell death. Broken cells release intracellular components to induce the increase of inflammatory mediators 17, such as IL-6, CCL4, TGF-β3 and VEGF 10.
In addition, sphingolipid metabolism participates in inflammatory reaction through various mechanisms. Sphingolipid metabolism can affect the activation of pro-inflammatory gene-NFB. Ceramide can activate TLR4 signal 18. In sphingomyelin synthase 2 knockout mice, toll like receptor 4-MD2 complex levels on the surface of macrophages were also significantly reduced 19. Exogenous addition of SM can directly trigger the inflammatory response of mouse macrophages in an NFB independent manner 18. Abnormal activity of sphingolipid metabolism can lead to the intensification of intraocular inflammation and eye tissue damage.
L-Palmitoylcarnitine is a long-chain acyl carnitine that accumulates in the muscle membrane during ischemia and disturbs the membrane lipid environment. It has been proved that acylcarnitine is a metabolite with biological activity and inflammatory properties. Palmitoylcarnitine increases the stress indicators of muscle cells, such as cell permeability, caspase-3 cleavage, IL-6 production, and MAP kinase pathway 20. When the concentration of L-palmitoylcarnitine increases, it can inhibit carnitine palmitoyltransferase activity 21, 22 and limit the transport of long-chain fatty acids in the mitochondria. More than 60% of retinal mitochondria are in photoreceptor cells 23. When mitochondrial dysfunction occurs, it may lead to the death of photoreceptor cells 24, and denaturation of neurons 25. After retinal detachment, the up-regulation of L-palmitoylcarnitine level caused by hypoxia in the inner retina may aggravate the damage of visual function. We also detected that many fatty acid metabolites were up-regulated in the eyes of RRDCD patients, such as 1-stearoyl-2-oleoyl-sn-glycerol 3-phosphocholine, 1-stearoyl-2-arachidonoyl-sn-glycerol, linoleic acid and 4,7,10,13,1 6,19-docosahexaenoic acid. The up-regulation of L-palmitoylcarnitine and various lipid metabolites may indicate that the retinal or choroidal lipid oxidation process in RRDCD patients was damaged 26. The retina is rich in lipids and requires high metabolism 27. lipid β-oxidation is an important pathway of retina obtaining energy, especially in RPE cells, muller cells and photoreceptor cells 23. Damaged energy pathway and the accumulation of long-chain fatty acids in cells can induce apoptosis 28. Previous studies have shown that when the oxidative utilization and storage of long-chain fatty acids are unbalanced, it can cause cellular stress and inflammation 29. Accumulation of long-chain fatty acids leads to the accumulation of reactive oxygen species (ROS) 30. ROS can induce inflammation as a metabolic stressor. It has been shown that the overproduction of ROS after retinal detachment causes oxidative stress, activates inflammasomes, and interacts with the inflammatory mediators, including IL-1β, TNF-α, and CCL2 31. Previous studies have shown that PVR is related to the increase of free radical formation and the decrease of antioxidant activity in human vitreous 32.
The limitation of this study is that non-target metabolites cannot be quantitatively to analyze metabolomics. Our results need to be verified by more experiments. However, our results revealed the possible pathways involved in RRDCD. The study helps us to explore the pathogenesis of RRDCD and find biomarkers.
In this study, UHPLC-Q-TOF-MS-based metabolomics identified 135 metabolites that were significantly changed in the vitreous fluid of RRDCD patients. Our results show that abnormal sphingolipid metabolism may aggravate cell migration and ocular inflammation, and promote the occurrence of PVR. The damaged retinal lipid metabolism may directly or indirectly lead to the death of photoreceptors. Our research provides clues and theoretical basis for exploring the pathogenesis of RRDCD. In future research, we plan to expand the sample size, conduct targeted quantitative analysis, and try to find upstream substances.

Ethics approval and consent to participate: This research followed the Declaration of Helsinki’s guiding principles and was approved by the Human Investigation Ethics Committee of Wuxi Second Hospital of Nanjing Medical University (protocol number: ChiCTR2000030296). All patients had provided informed consent before being included in the examination.
Consent for publication: Not applicable.
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Social Development Project of Jiangsu Provincial Science and Technology Department [grant number BE2017627].
Authors' contributions: LFY: analysis of data and wrote the paper. XCG: collect data. YHC: collect samples. SSL and ZFW: reviewed and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments: We thank Shanghai Applied Protein Technology Co., Ltd., for technological assistance.