Results
During the review process of pretreatment CT, one discordant case was discovered among 45 cases and was classified as cT3r after discussion. Following this review, 34 patients with cT3br tumors were chosen. Because one tumor became unresectable due to progressive disease with newly developed lung metastasis during NACRT, 33 patients who completed NACRT followed by MIE were included in the analysis. Figure 2 shows typical cT3br CT images in this study. Table 1 displays the clinical characteristics of the patients. The adjacent organs with suspected invasion included, the trachea/bronchus in 20 cases, followed by the aorta in 12, the spine in 2, and the pulmonary vein and subclavian artery in one each. Table 2 shows the short-term outcomes of NACRT followed by MIE. NACRT was completed in all cases without major complications. Seventy percent (23 out of 33) of patients received one cycle of chemotherapy. In terms of the MIE approach, TE was chosen in 20 cases, while RE was chosen in 13 cases. There was no conversion to open thoracotomy or postoperative mortality. Grade II or higher (Grade ≥ II) postoperative complications were observed in 11 (33%) cases and those of Grade III or higher (Grade ≥ III) in 7 (21%) cases. Table 2 shows the details of complications of Grade ≥ III, and the other Grade II complications included ileus (n = 1), cholecystitis (n = 1), pneumonia (n = 1), and surgical site infection (n = 1). Table 2also included pathological results. R0 resection was obtained in 32 (97%) of the patients. Regarding the histological pretreatment efficacy, 8 (24%) patients achieved Grade 3, and 4 (12%) patients achieved pathological complete response (pCR).
|
Variables |
n = 33 |
|---|---|
|
Age, median (range) |
66 (47–80) |
|
Sex, n, Male/Female |
22/11 |
|
SCC antigen before treatment (ng/ml), median (range) |
2.4 (0.5–21.3) |
|
Body Mass Index, median (range) |
19.5 (13.9–25.9) |
|
ECOG Performance Status, n (0/1/2) |
22/10/1 |
|
Tumor location (Ceb/Utb/Mtb/Ltb) |
1/10/20/2 |
|
Clinical Nc, n (N0/1/2) |
7/15/11 |
|
Clinical Stagec (II/III) |
7/26 |
|
Adjacent organ with suspected invasiond |
|
|
Trachea/Bronchus (invasion by MLNe) |
20 (7) |
|
Aorta (invasion by MLNe) |
12 (1) |
|
Spine |
2 |
|
Pulmonary vein |
1 |
|
Subclavian artery |
1 |
|
a esophageal squamous cell carcinoma |
|
|
b Ce; Cervical esophagus, Ut; Upper thoracic, Mt; Middle thoracic, Lt; Lower thoracic |
|
|
c Determined by the 8th edition of the AJCC TNM classification |
|
|
d contains duplicate organs, e metastatic lymph node |
|
|
Variables |
n = 33 |
|---|---|
|
Number of chemotherapy cycles, n, (1/2) |
23/10 |
|
Response of neoadjuvant chemoradiotherapy |
|
|
Non-target lesions (CRa/IR,SDa) |
2/31 |
|
Target lesions (PRa/SDa) |
4/3 |
|
Approach for esophagectomy, n, (Thoracoscopic/Robotic) |
20/13 |
|
LN dissection, n, (2 field/3 field) |
14/19 |
|
Reconstruction route, n, (Posterior mediastinal/Retrosternal/Intrathoracic) |
20/12/1 |
|
Total operative time, min, median (range) |
549 (412–846) |
|
Thoracoscopic time, min, median (range) |
232 (156–399) |
|
Blood loss, g, median (range) |
50 (0–680) |
|
Hospital Stay, days, median (range) |
23 (11–137) |
|
Postoperative complicationb, ≥ Grade II, n (%) |
11 (33) |
|
Postoperative complicationb, ≥ Grade III, n (%) |
7f (21) |
|
Recurrent laryngeal nerve palsy |
4 |
|
Tracheoesophageal fistula |
2 |
|
Lymphorrea |
1 |
|
Anastomotic leakage |
1 |
|
Pneumonia |
1 |
|
Pathological Tc, n (T0d/T1a/T1b/T2/T3) |
8/1/1/3/20 |
|
Pathological Nc, n (N0/1/2/3) |
8/17/6/2 |
|
Pathological Stagec (0e/IIA/IIB/IIIA/IIIB/IVa) |
4/1/9/1/16/2 |
|
Residual tumor, n, (R0/1/2) |
32/1/0 |
|
Pretreatment efficacy, Grade, n, (1a/1b/2/3) |
9/5/11/8 |
|
Adjuvant chemotherapy, n, (None/S-1/5-FU + Cisplatin/Nivolumab) |
15/7/6/5 |
|
a CR; Complete Response, PR; Partial Response, IR; Incomplete Response, SD; Stable Disease, |
|
|
b by Clavien-Dindo classification, c Determined by the 8th edition of AJCC TNM classification |
|
|
d No residual tumor cells, e pathological complete response, f contains duplicates, |
|
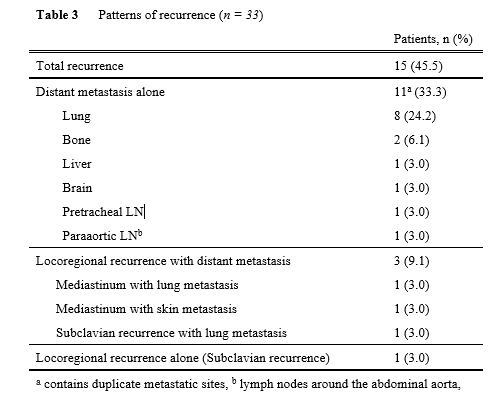
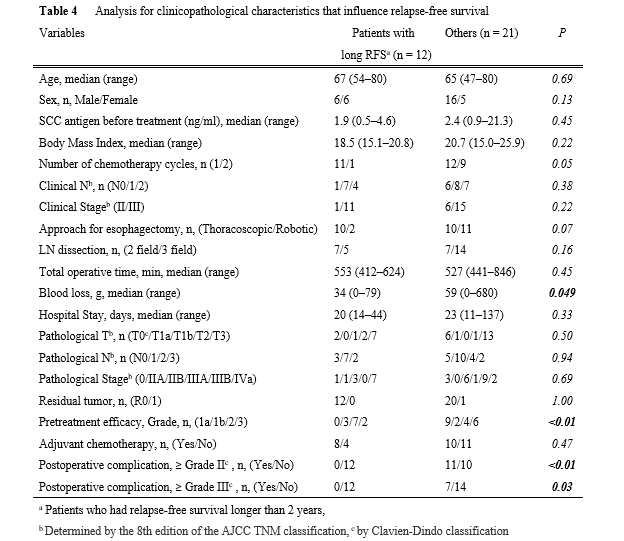
Next, mid-term oncological outcomes were assessed (Table 3 and Fig. 3). Figures 3a and b show the RFS and OS curves. The 2-year RFS/OS in all populations was 54%/73%, respectively. With a median follow-up of 675 days, recurrence was found in 15 cases (45.5%), with 14 (93%) recurring within 2 years (Table 3). Regarding the recurrence pattern, distant metastases alone were found in 11 cases (33.3%). Three cases (9.1%) had locoregional recurrences with distant metastasis, while only one case (3.0%) had locoregional recurrence alone. To determine which factors influenced relapse-free survival, the clinicopathological characteristics of 12 patients with RFS longer than 2 years (long RFS, n = 12) were compared to those of the other patients (n = 21) (Table 4). Long RFS patients had considerably better histological pretreatment efficacy than the others (p < 0.01). Interestingly, postoperative complications were significantly lower in patients with long RFS compared to other patients. Postoperative complications of Grade ≥ II or ≥ III were never observed in patients with long RFS, whereas those of Grade ≥ II or ≥ III were observed in 11 (52%), and 7 (33%), respectively, of the other patients. The significant difference was confirmed regardless of whether the complication was of Grade ≥ II (p < 0.01) or Grade ≥ III (p = 0.03) was selected for the analysis. Finally, the clinicopathological characteristics of patients with and without postoperative complications (Grade ≥ II) were examined (Supplementary Table 1). Male patients (p = 0.04), longer operation time (p = 0.03), and longer hospital stays (p < 0.01) were associated with postoperative complications. Figures 3c and d depict RFS and OS curves with and without postoperative complications (Grade ≥ II). RFS in patients with complications was significantly lower than in patients without complications (p = 0.049) (2-year RFS: 0% and 65%, respectively). There was no significant difference in OS (p = 0.35) (2-year OS: 65% and 74%, respectively).