Methods
Participants: This study was conducted on students who were studying in Jahrom University of Medical Sciences.
Sample size estimation: There was no previous comparable study in Ferula on which to base data for a Sample size calculation, then based on FG score results in another similar article (18) with this formula:
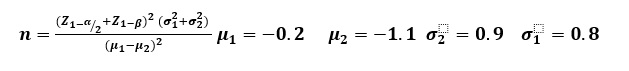
And significance level of 0.05, a power level of 0.80, with a 10% attrition rate investigated that 18 samples should enroll to study. Due to the samples available to, 17 samples were included in the study.
Inclusion criteria: In this study, the participants were young girls aged 18-30 years that diagnosed PCOS based on Rotterdam criteria (table 1)

The exclusion criteria were medical conditions such as androgen-secreting tumors, hyper prolactinemia, thyroid disorders and Cushing’s syndrome (determined by suitable laboratory assay), pregnancy and lactation.
Random allocation: simple or finite random sequences with table of random number was Created. Based on this step, each participant was given a unique identifier. In concealment random allocation step, boxes were coded of identical shape and size numbered randomly containing herbal medicine or placebo. Based on this step, participants assigned randomly to one of two groups. Each Participant in intervention group received active compounds in capsular form (each capsule contained 100 mg of oleo-gum resin of Ferula assa-foetida) twice daily for 3 months. In the same way, participants in placebo group received capsules of 100 mg prepared from oral paraffin.
Blinding: The participants, and all investigator, were unaware of the capsule contents. After 3 months interventions, the contents of the capsules given to each group were revealed by the pharmacist. After study was completed, the researchers received information about the numbers and the nature of each drug.
In the execution of a randomized specialized process, S.A is the person who created the random sequence, F.GH is person who has evaluated and registered the samples in terms of include and exclude criteria. F.SH is the person who assigns the samples to the groups. M.T is the who, analyzed of data.
Preparation of Capsules: Root and Stem of Ferula assa foetida L. (gum) was purchased from a medicinal plant market (Adonis Gol Darou), a supplier of herbal medicines,Tehran, Iran. In Department of phytopharmaceuticals (Traditional Pharmacy), School of Pharmacy, Shiraz University of Medical Sciences with Herbarium Voucher Number 981, Ferula assa foetida essence was produced by distilling the Root and Stem with steam and then for this study they were formed into pearl-shaped pills (100 mg). The major components of Ferula assa foetida included 65% oleo g um resin, 20% essence, 25% stem(19).Ferula assa foetida drug toxicity was assessed in a past study and its safety had been reported, Based on systematic review study, Ferula may be cause the skin allergic sign and do not recommended for infant(12).
The placebo contained 100 mg paraffin. In order to isotropy, each pearl was placed inside a capsule and was coded.
Data Collection: The primary outcome was clinical parameters (BMI, FG score, Menstruation periods), that data were obtained at the beginning of the study and at the end of the study period. Secondary outcome were hormonal (Dehydroepiandrosterone sulfate (DHEAS), Free testosterone (FT), Follicle-stimulating hormone (FSH), Luteinizing hormone (LH) and Prolactin(PRL)) and ultrasound parameters (Endometrial thickness, ovarian volume and Number of follicle in both Ovary). A venous blood sample was obtained from the studied population to evaluate the changes in the level of biochemical factors. Plasma was separated and kept at -20ºC until it was assayed for DHEAS, FT, FSH, and LH using immunoassay kits (Monobind Inc, CA, US) according to the manufacturer’s instructions.
Testosterone, DEHAS, Prolactin, TSH, FSH and LH were measured by using the enzyme-linked immune sorbent assay method using the Eliza Kit (Monobind Inc., Germany).
In all groups, Participants underwent Trans abdominal ultrasound for evaluating ovarian volumes, number of follicles of both ovaries, and endometrial thickness at 4.4-MHz (Alpinion, Seoul, South Korea) by a one sonographer in the follicular phase of menstrual cycle except in participants with oligo or amenorrhea
Ethics Consideration: The study was approved by the Ethics and Research Committee of Jahrom University of Medical Sciences (reference number: ums.REC.1393.023) and registered in the Iranian Randomized Clinical Trial (IRCT2016040427207N1).
Statistical Analysis: All statistical analysis was performed using SPSS statistical software version 14 and Graph pad Prism (version 6). Quantitative variables were reported as mean and standard (95% confidence Interval). The normal distribution of the data was tested by Kolmogorov–Smirnov test and a non-parametric test was conducted to evaluate the objectives. clinical, hormonal and ultrasound parameters were analyzed by using Man Whitney tests to intergroup comparison before and after 3 months and Wilcoxon test for comparison between groups before and after 3 months interventions.