Methods
Patient samples and cell culture
In total, 87 cases of LSCC detected between 2015 and 2017 in the second department of Jilin University First Hospital were examined in the study. The female to male ratio was 3.14:1. Sixty-one cases involved lymph node metastasis (Table 4), and 77 cases involved invasion of adjacent tissues. This study was approved by First Hospital Of Jilin University Institutional Review Boards. All methods were carried out in accordance with relevant guidelines and regulations.The informed consent was obtained from all patients, if the patients are under 18, from a parent.
Hep-2 and A549 cells [36] were provided by the Key Laboratory of Pathology and Biology of the Ministry of Education of Jilin University. The cells were cultured in H-DMEM containing 10% fetal bovine serum in an incubator containing 5% CO2 at 37°C.
Immunohistochemistry
Immunohistochemical staining was performed to analyze the expression of AKR1B10, Ki-67, MTp53, and MMP2 in LSCC and adjacent tissues. Tissue sections were incubated at 70°C for 1 h, followed by incubation with 100% xylene for 10 min, 100% ethanol twice for 5 min each, and then 95%, 90%, and 80% ethanol for 2 min each. The slides were washed and repaired under high pressure. After setting up and running the automatic immunohistochemical stainer program, slides were counterstained, differentiated, subjected to anti-blue staining, dehydrated, and cleared. Slides were finally observed under a microscope.
Result interpretation
AKR1B10 and MMP2 staining intensity was scored as the percentage of positive cells as follows: <5%, 0 points; 5%–10%, 1 point; 11%–50%, 2 points; and >50%, 3 points Meanwhile, the staining color was scored as follows: no color, 0 points; light yellow, 1 point; dark yellow, 2 points; and yellowish brown, 3 points. The staining score was calculated by summing the intensity and color scores and categorized as follows: <2, negative; 2–3, weakly positive; 4–5, moderately positive; and ≥6, strongly positive [17].
For Ki-67 scoring, the percentage of positive cells among 1000 positive cells was categorized as follows: ≤25%, low expression; and >25%, high expression [37].
MTp53 expression was scored as the percentage of positive cells among 1000 cells as follows: ≤10%, negative; and >10%, positive [38].
Reverse-transcription polymerase chain reaction (RT-PCR)
Ribonucleic acid (RNA) was extracted from Hep-2 and A549 cells. RNA was reverse-transcribed into cDNA according to the instructions of the kit. After the PCR reaction system was thoroughly mixed, 30 cycles were performed according to the reaction conditions.
For electrophoresis, 10 μl of the PCR product were added to each well and electrophoresed at 100 V for 1 h, and the results were observed using an imager.
Western blotting
To extract protein, Hep-2 and A549 cells were washed with cold phosphate-buffered saline (PBS), after which the culture dishes were placed on ice. Cells were lysed with 500 μl of lysate per dish for 30 min, and cells were scraped into Eppendorf tubes and centrifuged at 15 × 10 rpm for 15 min. The supernatant was retained and used to measure the protein concentrations in the samples.
For electrophoresis, 30 μg of the protein sample were added to each well, electrophoresed at 80 V for 30 min, and then electrophoresed at 120 V for 90 min. Proteins were then transferred to a membrane and incubated sequentially with primary and secondary antibodies.
Immunofluorescence staining
Hep-2 and A549 cells were cultured in 24-well plates. After permitting adherent growth, cells were fixed with paraformaldehyde for 20 min, washed three times with PBS, incubated with 0.3% Triton for 5 min, and washed three times with PBS. Cells were then exposed to 0.5% BSA for 30 min. After aspirating the liquid, cells were incubated with 0.1% BSA-diluted AKR1B10 antibody (1:50) overnight at 4°C and washed three times with PBS. Cells were incubated with the fluorescent secondary antibody (1:400) at room temperature for 1 h, washed three times with PBS, stained with DAPI for 10 min, and washed with PBS, and the stained cells were observed under a fluorescence microscope.
Effect of OA on the activity and expression of AKR1B10 in Hep-2 cells
To conduct ELISA, 6 × 105 Hep-2 cells were added to each well of a six-well plate. After permitting adherent growth, cells were cultured with 0, 10, 30, or 60 μM OA for 48 h and lysed (approximately 1 million cells/ml). Cells were then centrifuged (3 × 1000 rpm, 10 min). According to the AKR1B10 enzyme activity assay kit instructions, a standard curve was drawn to calculate enzyme activity. For RT-PCR and Western blotting, cells were treated with OA, after which RNA and protein were extracted. AKR1B10 mRNA and protein levels in cells were detected via RT-PCR and Western blot, respectively.
Based on the results of the aforementioned experiments, 30 μM OA was selected for the subsequent experiments.
CCK8 experiment
Hep-2 cells were cultured in seven 96-well plates at 2000 cells/100 μL per well. Three replicates each were created for the control and experimental groups. The control group was treated with H-DMEM containing 10% serum. The experimental group was treated with H-DMEM containing 30 μM OA. Cell growth was measured continuously for 7 days by adding 10 μL of CCK8 solution at 37°C for 1–4 h, measuring the absorbance at 450 nm, and plotting the proliferation curve.
Scratch test
A straight line was drawn on the back of a six-well plate. Hep-2 cells (6.5 × 105) were added to each well and cultured overnight. Then, the cell monolayer was scratched in a direction perpendicular to the line. The control group was cultured in serum-free H-DMEM. The experimental group was cultured in serum-free H-DMEM containing 30 μM OA. Cell migration was observed under a phase contrast microscope at 0 and 48 h. Cell migration was calculated using the following formula:
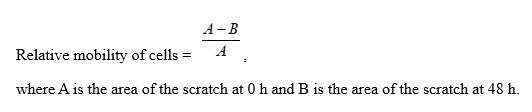
Transwell experiment
Initially, 65 μL of diluted Matrigel were added in the upper chamber (Matrigel:H-DMEM ratio = 1:5). Then, Hep-2 cells (7 × 104 cells/200 μL) cultured in serum-free H-DMEM for 12 h were added to the upper chamber. The experimental group was cultured in H-DMEM containing 30 μM OA, and the control group was cultured in H-DMEM. Meanwhile, 500 μL of H-DMEM medium containing 15% serum were added to the lower chamber in both groups. Cells were incubated for 48 h in a 5% CO2 incubator at 37°C. The culture medium in the upper chamber was discarded, and the cells in the upper chamber were cleaned with a cotton swab and fixed with paraformaldehyde for 20 min. After staining with crystal violet, the cells were observed under an inverted microscope.