Methods
Between December 2018 and June 2020, we consecutively recruited 25 patients fulfilling the diagnostic criteria for VM [5]. All the VM patients were evaluated in the intercritical phase and in absence of any actual pharmacological prophylactic treatment. All patients also underwent a complete vestibular evaluation, which involves bedside examination, caloric reflex test, the video head impulse test (vHIT) and functional head impulse test (fHIT). In the same period, we recruited 20 patients suffering from AUV. We diagnosed AUV as a syndrome characterized by rapid onset of severe dizziness without neurologic or audiologic symptoms, unidirectional horizontal nystagmus, unilateral vestibular areflexia/hyporeflexia on bithermal caloric test, and positive head impulse test result in the direction opposite to the fast phase of the nystagmus. Each patient with AUV underwent to the vestibular instrumental examination (bithermal caloric test and video Head Impulse Test, vHIT) after the disappearance of the acute symptomatology within 8-14 days after the onset of the symptoms. The vHIT was performed by employing a dedicated device (ICS Impulse System; GN Otometrics): the patient was asked to stare at an Earth-fixed target (3-cm-diameter spot located 1.5 m in front); then, 20 low-amplitude (10°-20°) but high-velocity head impulses (150-200 deg/s) were randomly administered on each side for every semicircular canal. The device software automatically calculates the average high-velocity VOR gain. The software also calculates the asymmetry index (AI%, normal values within 15% [% confidence limit 0-15%]; this value resulted from our own data collected on a group of 50 normal subjects age ranging 20 to 80) between the right and left sides. AI was calculated as 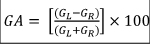 where GR denotes right sided mean gain and GL denotes left sided mean gain. The AI% shows differences between the sides in terms of high-velocity VOR [17]. All the patients underwent to cranial MRI investigation to rule out a central nervous system involvement. Patients suffering from Menière’s Disease, sudden sensorineural hearing loss or recent head trauma were excluded. Altogether we considered a control group consisting of 20 healthy subjects, excluding subjects who suffered in the past from AUV and who did report recent episodes of benign paroxysmal positional vertigo. They also must not report a personal or familiar history of migraine. All the subjects underwent to fHIT using the Beon Solution System® (Beon Solution srl, Treviso, Italy): the patient was seated 1.5 meters away from the computer screen (Full HD, resolution 1920x1080, refresh rate 60 Hz) and was wearing an accelerometer mounted on the forehead through a tape. Landolt's Optotype C was used and first of all the examination acuity was evaluated, progressively decreasing the size of the symbols: in this way the dimensions of the visual stimulus were normalized according to the characteristics of the subject examined. Subsequently, the examiner quickly moves the patient's head on the horizontal plane (at least ten turns in each direction), with an acceleration ranging between 3000 and 6000 °/s2. During the movement (as soon as the acceleration reached a limit set by the manufacturer) a symbol was displayed on the monitor for a time of 33 milliseconds [18]. The patient was asked to recognize the symbol that appeared on the monitor during head movement and to press the equivalent key. The parameter taken into consideration was the average percentage of correct answers (%CA) with respect to the total of answers presented for each frequency band examined (acceleration bins). The test was then repeated with the addition of confounding screen (CS) that is a moving background constituted by a rotating cloud of yellow dots, named optokinetic fHIT (o-fHIT) displayed on the screen while the above-described test is being performed. To evaluate the results of the test, the following procedure was carried out:1) the answers for the head rotation to the right and those for the rotation to the left were separated and considered different tests: in this way the number of exams becomes double compared to the number of patients; 2) For each test, the difference in %CA without and with confounding screen was evaluated.
where GR denotes right sided mean gain and GL denotes left sided mean gain. The AI% shows differences between the sides in terms of high-velocity VOR [17]. All the patients underwent to cranial MRI investigation to rule out a central nervous system involvement. Patients suffering from Menière’s Disease, sudden sensorineural hearing loss or recent head trauma were excluded. Altogether we considered a control group consisting of 20 healthy subjects, excluding subjects who suffered in the past from AUV and who did report recent episodes of benign paroxysmal positional vertigo. They also must not report a personal or familiar history of migraine. All the subjects underwent to fHIT using the Beon Solution System® (Beon Solution srl, Treviso, Italy): the patient was seated 1.5 meters away from the computer screen (Full HD, resolution 1920x1080, refresh rate 60 Hz) and was wearing an accelerometer mounted on the forehead through a tape. Landolt's Optotype C was used and first of all the examination acuity was evaluated, progressively decreasing the size of the symbols: in this way the dimensions of the visual stimulus were normalized according to the characteristics of the subject examined. Subsequently, the examiner quickly moves the patient's head on the horizontal plane (at least ten turns in each direction), with an acceleration ranging between 3000 and 6000 °/s2. During the movement (as soon as the acceleration reached a limit set by the manufacturer) a symbol was displayed on the monitor for a time of 33 milliseconds [18]. The patient was asked to recognize the symbol that appeared on the monitor during head movement and to press the equivalent key. The parameter taken into consideration was the average percentage of correct answers (%CA) with respect to the total of answers presented for each frequency band examined (acceleration bins). The test was then repeated with the addition of confounding screen (CS) that is a moving background constituted by a rotating cloud of yellow dots, named optokinetic fHIT (o-fHIT) displayed on the screen while the above-described test is being performed. To evaluate the results of the test, the following procedure was carried out:1) the answers for the head rotation to the right and those for the rotation to the left were separated and considered different tests: in this way the number of exams becomes double compared to the number of patients; 2) For each test, the difference in %CA without and with confounding screen was evaluated.
All the subjects were submitted to the Italian version of the Situational Vertigo Questionnaire (SVQ) [19] to identify if vertigo symptoms are provoked or exacerbated by specific disorienting visual context. The total score will be then calculated as the sum of single item scores divided per 19 minus the number of never experienced situations (total score/19-number of “never experienced” answer).
Ethical review and approval by the local Institutional Board (Comitato Etico Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy) were waived for this study. Due to its retrospective nature, it not being set up as part of a research project. Furthermore, the study does not include new experimental diagnostic protocols and the patients included in the study were diagnosed according to national guidelines. Written informed consent was obtained from all participants, and the study was conducted in accordance with the 1964 Declaration of Helsinki.
Statistical study
The data obtained were statistically compared with the variance analysis method. The values obtained are distributed in a normal way, therefore it was possible to perform:
1) a study of variance (one-way ANOVA), which allowed to reject the null hypothesis of homogeneity of the groups (p <0.05).
2) A statistical comparison between patients with migraine vertigo (one-way t-test) and normal group;
3) A statistical comparison between patients with compensated acute vestibular deficit and the normal group (one-way t-test);
4) A comparison between patients suffering from migraine and those suffering from compensated acute vestibular deficit (one-way t-test).
A study of variance with Tukey's correction was also carried out, which was also significant due to the rejection of the null hypothesis between the groups (p <0.05).