Results
Safety. The objective of this Phase I study was to obtain preliminary data, in an open-label study, on the safety of Aviptadil, infused iv. (50 or 100 pmol/kg/hr, infusion time 6 to 12 hr) in patients with ARDS induced by sepsis. In total, 8 patients were included in the study. Three patients progressed to the high dose group while infusion in the remaining 5 patients was maintained at the lower dose. In 5 cases, there was surgery preceding the infection. Consistent with the polymorbidity of the patients admitted to the trial, they also received a large number of concomitant medications and other supportive measures.
In the lower dose group, Aviptadil administration was stopped in one (bronchial obstruction due to hypersecretion considered to be unrelated to Aviptadil) and the dose halved in another (Hypotension). In the high dose group with 100 pmol/kg/hr (3 patients) Aviptadil administration was stopped in none. However, dose was transitorily reduced in two, due to hypotension (1 case) or to bigeminy (1 case). Watery stools were observed in one patient in the high dose group.
Two patients died during the long-term follow-up period. Patient # 5, aged 87 years, male, suffered was successfully extubated and discharged from the ICU, but succumbed to a massive right-sided middle cerebral artery infarct three weeks after VIP administration. Supportive measures were stopped at the request of the family. Patient # 2, aged 80 years, male, received the Aviptadil infusion for only 2 of the intended 6 hours because oxygen saturation dropped to 85%. The patient at this point was suctioned from the endotracheal tube and ventilated with an AMBU bag + bronchodilator nebulizer treatment. The endotracheal tube yielded large amount of thick, purulent secretions. The arterial oxygen saturations improved to baseline of 90-92%, but the Aviptadil infusion was not restarted. He died 4 days later; the death was considered a direct consequence of underlying sepsis and unrelated to VIP infusion.
Figure 1. VIP dosing per patient over time
Table 1 gives an overview of the adverse events observed in these severely diseased patients. It is very likely that the adverse events of hypotension and diarrhea are attributable to Aviptadil after IV administration.
Table 1. Adverse events and outcomes following administration of IV infused VIP.
|
Patient No. |
AEs |
Description of Adverse Event |
Severity |
Serious |
Unexpected |
Course |
Relationship to Aviptadil |
|
CRF # 1 |
No |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
|
CRF # 2 |
Yes |
Hypoxemia, acute |
Severe |
Yes |
No |
N.A. |
Unlikely |
|
Death |
Severe |
Yes |
N.D. |
N.A. |
No |
||
|
CRF # 3 |
Yes |
Hypotension |
Mild |
No |
No |
disappeared after intervention |
Possible |
|
CRF # 4 |
Yes |
Seizures |
Severe |
Yes |
yes |
N.D. |
No |
|
CRF # 5 |
Yes |
Bigeminy |
moderate |
No |
No |
disappeared after intervention |
Probable |
|
Diarrhea |
Mild |
No |
Yes |
Spont. disapp. |
Probable |
||
|
Death |
Extreme |
Yes |
No |
N.A. |
No |
||
|
CRF # 6 |
Yes |
Seizures |
N.A. |
No |
Yes |
N.D. |
No |
|
CRF # 7 |
No |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
N.A. |
|
CRF # 8 |
Yes |
Hypotension |
moderate |
No |
No |
disappeared after intervention |
Probable |
|
Pneumothorax |
Severe |
yes |
yes |
disappeared after intervention |
No |
Clinical Outcome
Clinical vignettes were available for seven of eight treated patients (see supplemental material). Examination of patient vignettes reveals multiple comorbidities that are typically seen in patients who develop inpatient sepsis and progress to ARDS. Prior to treatment with VIP, patients received maximum tolerated medical therapy and were on mechanical ventilation. As seen in Table 2, patients had ARDS with moderate to poor prognosis based on PaO2/ FiO2 ratio. All patients demonstrated improvement in oxygenation and reduced evidence of respiratory distress (see supplemental material). Seven patients were successfully extubated and discharged from the ICU. The clinical team was encouraged by improvement in the respiratory status of patient MA and believed it to be consistent with survival. However, the underlying neurologic condition in light of the patient’s end of life directives caused the family to withdraw consent for further care.
Table 2: Baseline respiratory distress vs. clinical outcome
|
Patient Initials |
PaO2 |
FiO2 |
PaO2/ FiO2 |
Clinical outcome |
|
Patient 1 |
84 |
0.24 |
350 mmHg |
Survived |
|
Patient 2 |
83 |
0.8 |
104 mmHg |
Survived |
|
Patient 3 |
45 |
0.9 |
50 mmHg |
Survived |
|
Patient 4 |
115 |
0.7 |
164 mmHg |
Life support withdrawn |
|
Patient 5 |
59 |
0.7 |
84 mmHg |
Survived |
|
Patient 6 |
86 |
0.5 |
172 mmHg |
Survived |
|
Patient 7 |
86 |
0.88 |
98 mmHg |
Survived |
|
Patient 8 |
Missing data |
Missing data |
Missing data |
Survived |
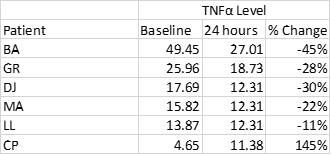
Measurement of TNFα
As an exploratory endpoint, Tissue Necrosis Factor α (TNFα) was measured in 6 of 8 patients. In five of those patients, a modest decrease in TNFα was seen, while patient #6 demonstrated a substantial increase from baseline (Table 2)
Table 2: TNFα level at baseline and 24 hours post VIP infusion