Methods
Participants
Twenty patients with stroke were recruited from within a community self-help group for stroke, using convenience sampling. The inclusion criteria were: (1) stroke with unilateral hemispherical involvement; (2) aged 18 or above; (3) stroke onset of six months or more before the study; (4) able to understand verbal instructions and follow one-step commands; (5) no excessive spasticity, defined as a score of 2 or higher on the Modified Ashworth Scale [20]; (6) no complaints of excessive pain or swelling over the paretic upper extremity; (7) no prior participation in any experimental or drug studies within one month; and (8) able to understand the meaning of the study and give informed consent to participate. The choice of a post-stroke period of six months or above as an inclusion criterion for patients with chronic stroke reflected the probability that any spontaneous recovery would have slowed down by the time of the study, thus enabling more brain reorganization in response to the therapeutic intervention under study. Before commencement of the study, written and informed consent was obtained from all patients. The study was carried out in accordance with the principles of the Declaration of Helsinki. Ethical approval was sought and obtained from the Human Ethics Committee of the Hong Kong Polytechnic University (Ref. no.: HSEARS20180503002).
In order to investigate which level of severity of upper extremity impairment would benefit more from the treatment, patients were further stratified into two groups according to whether their hemiparetic upper extremity functional impairment was severe to moderate or mild: Group 1, the lower functioning group, included eight patients with severe to moderate impairments to a hemiparetic upper extremity (i.e., FTHUE level 1–4) who were able to move with bilateral arms, to demonstrate a mass flexion pattern in the shoulder/elbow, then an active extension control of the shoulder/elbow; Group 2, the higher functioning group, included 12 patients with moderate to mild hemiparetic upper extremity impairments (i.e., FTHUE level ≧ 5) who were able to demonstrate the beginnings of an ability to combine components of strong mass flexion and strong mass extension patterns, and who were able to perform some release of the hand, as well as isolated control of forearm and hand with fair strength.
Task-specific virtual reality training program (TS-VR)
In stage 1, we developed the TS-VR using Unity3D (version 2017.3.1f1) and the LMC (version 3.2.1) as input devices for capturing real-time 3D positioning of the hand. The computer programming language C# was used in the development of the training programming. The TS-VR consists of seven general hand function tasks involved in ADL. These are designed according to the severity of the specific upper limb impairment of stroke patients, with reference to the levels 1–7 in the Functional Test for the Hemiplegic Upper Extremity (FTHUE) [21, 22]. The standardized tasks in the program for each level of impairment were carried out by the performing of discrete movements used in functional activities. These included bilateral and unilateral use of both hands, range of motion tasks, pronation and supination, and grasp and release (e.g., pouring water from a bottle) with the affected upper limb. The training tasks used in the TS-VR program and the corresponding levels (1–7) in the FTHUE are: Level 1 - Pushing a door with both hands (non-affected hand holding affected forearm); Level 2 - Picking up a cup from affected side of the table and moving to non-affected side; Level 3 - Holding a pouch in a static position for 15 seconds; Level 4 - Picking up a cup from non-affected side of the table and moving to affected side; Level 5 - Picking up a water bottle and pouring water into a cup; Level 6 - Picking up a book and placing it on a bookshelf; and Level 7 - Pressing the password pad. Figure 2 shows a screenshot of selected training tasks used in the training program, such as pushing open a door, pouring water, and pressing a button.
Unity3D is a cross-platform game engine that has been widely adopted by various industries for the development of VR programs, augmented reality (AR) games, computer simulations, and other experiences. Figure 3 shows the workflow of the development process for the TS-VR program. The 3D virtual environment and models are first created using SolidWorks. The models are then exported to FBX format and imported to the gaming engine. The imported models are usually gray in color, and the Unity engine supports and provides a physically based rendering material system to create realistic textures and color in various virtual scenes. Custom textures like wood and bricks can be added to the 3D models. The engine also provides a user-friendly interface for the adjusting of material values and parameters, such as “Metallic” and “Smoothness”. This enables the creation of a natural virtual environment under different lighting in real time.
The LMC is used to capture the 3D position of the hand, including the wrist and fingers. The LMC has a one-meter hemispherical view area with a 150 degrees field of view, and it can track users’ finger movements at a rate of over 300 frames per second, such that the screen display changes continually along with the user's every movement. The LMC uses structured infrared light emitters to create a 3D depth map with two cameras. The cameras capture data in the form of grayscale stereo images and process this data by extracting tracking information such as the position of wrist and fingers. The tracking algorithms then interpret the 3D data and infer the positions of the hand [23]. Using the LMC, the feature points in the wrist and fingers are extracted to interpolate the motion of the hand.
The LMC works using the Cartesian coordinate system in millimeters (mm). At the beginning, the origin is located at the middle of the device. These coordinates are converted to screen coordinates depending on the screen size or window size of the application, calculated by Eq. 1 [24]:
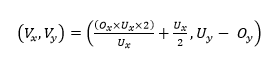 (1)
(1)
,where Vx and Vy are the x- and y-coordinates with respect to (w.r.t.) the screen coordinates; Ux and Uy are the width and height of the window; and Ox and Oy are the x- and y-coordinates w.r.t. the LMC coordinates.
To train the Hemiplegic Upper Extremity functions of the patients, interactions between different virtual models in the TS-VR training program are required. For instance, when patients push a door or press the password pad, the door will open and the password will display on the screen. In this case, a collider defined by common primitives in the space of the virtual objects is used to determine the interaction with the patients. A collider is a bounding box (Fig. 3) surrounding the virtual models. An interaction is triggered when the distance between the models and the finger is smaller than the distance threshold. Figure 1b shows the pseudo code of the pressing password pad task. In this task, the password is randomly generated. When the virtual hand model triggers the collider, the corresponding password number will be recorded and compared with the generated password. The task ends when the password is correct; otherwise, this task will restart. Similarly, other tasks, such as holding a handbag or placing an object also need to trigger the collider, and then a check is done to determine whether the task has been completed.
Rehabilitation and assessment
In stage 2, we sought qualitative comments for the TS-VR from an expert panel, composed of 10 occupational therapists, each with at least three years of experience in stroke rehabilitation. The panel interviewed 10 clients with stroke of various impairment levels recruited by convenience sampling in the community. After the interview, object component, program structure, and demonstration videos of the program were modified according to the comments of the panel, but the VR tasks remained similar in terms of content and difficulty.
In stage 3, we investigated whether a two-week program using TS-VR training would promote recovery of the paretic hand in patients with chronic stroke, using a single group longitudinal design study. Twenty patients were invited to participate in the TS-VR at the university laboratory. There were a total of 10 sessions, each 30 minutes in length, held five days per week over two consecutive weeks.
The equipment was set up as shown in Fig. 1. The LMC was installed on the table located in front of the monitor. The patients were seated in front of the computer and provided with enough space for them to move the upper extremity in order to complete the TS-VR tasks. The tasks were displayed on the computer monitor in front of the patients. The study was carried out under the one-to-one supervision of an occupational therapy student who was one of the investigators and had undergone training in using the TS-VR. Patients were instructed to sit on the chair, or else on their own wheelchair. The LMC was placed on another chair, in front of the participant and at an appropriate distance to ensure best sensitivity. In the first session, demonstrations were given by the investigator for each level of the TS-VR training tasks to ensure that patients understood how to perform the task correctly. In the following training sessions, the training regimens were tailored to the needs of the patients by adjusting the modes of training (i.e., the progressive and repetitive mode, respectively). For progressive mode, patients were instructed to perform the TS-VR tasks from level 1 to the highest level they could attain. For repetitive mode, patients were instructed to perform a specific task level repetitively for 5–10 minutes. Both progressive mode and repetitive mode were used in each 30 minutes training session, depending on patients’ needs, performance, and fatigue level. Patients with lower functioning (FTHUE of 1–4) were allowed to perform the TS-VR training task level 1 or 2 with the assistance of the non-affected hand.
At the beginning, during, and after each training session, patients were allowed to take a rest and do stretching exercises to relieve any tightness, tiredness, or discomfort of their paretic upper extremity. During the training, verbal and tactile cues were given by the investigator to correct patients’ abnormal posture and associated movement when they were performing the TS-VR tasks. Visual and audio cues which were built-in to the TS-VR also provided sensory feedbacks to the patients. The time and error records after each completion were also recorded in order to provide immediate feedbacks to the patients.
Outcome measurements
Outcome measurements were conducted at baseline (pre-treatment, first session, week 0), post-treatment (last session, week 2), and follow-up (week 5) at the university laboratory. The primary outcomes were two laboratory-based assessments for arm functions, including the Fugl-Meyer Assessment-Upper Extremity score (FMA-UE) [25, 26] and the Wolf Motor Function Test (WMFT) [27]. The FMA-UE [28] is a well-known impairment test which evaluates patients with stroke through selective movements. The upper limb subscore consists of 32 items, each of which represents movement components, scored on a three-point scale (0, 1, 2). A higher score denotes a better recovery. The WMFT was chosen because the score range of this test is relatively greater and therefore more suitable for capturing both higher and lower levels of arm functioning in stroke patients [29]. The secondary outcome was a self-reported questionnaire, the Motor Activity Log (MAL) (including the Amount of Use scale (AOU) and the Quality of Movement scale (QOM)), indicating how often and how well patients used their affected arm in ADL [30]. It was administered for the actual arm use of the affected hand in daily life. It was carried out by asking patients about the use of their hemiparetic upper limb in 30 daily living activities over one week. Both subscales in the MAL - the AOU and the QOM – were shown to the patients while going through each item over the course of a semi-structured interview.
Statistical analysis
Data analysis was performed using SPSS. Baselines, including demographic data and pre-treatment scores for the outcome measures, were calculated according to descriptive statistics. Nonparametric tests were used because the data collected was either categorical or because sample size was small. The Friedman Test was used to evaluate any significant difference in the hand functions and arm use of patients before and immediately after the training and at within-group follow-up sessions. The Wilcoxon Signed-Rank Test was used for post hoc analysis, in which the measurements taken for each variable on the three occasions (i.e., before and immediately after the training, and at follow-up) were compared to determine where any significant differences lay. The level of significance was set at p ≦ 0.05 for the Friedman Test after Bonferroni adjustment (0.05/n; n = number of tests). The Friedman Test and the Wilcoxon Signed-Rank Test were later used for separate analyses when the patients were stratified into lower-functioning and higher-functioning groups according to the severity of their hemiparetic arm functional impairment.