2. Materials And Methods
2.1. Reagents and materials
Deuterium oxide (D2O, 99.9 atom % D) containing 3-(trimethylsilyl) propionic-2, 2, 3, 3-d4 acid sodium salt (TSP, 0.05 wt%) was purchased from Sigma-Aldrich, Co. (St Louis, MO, USA) for 1H NMR analytical sample preparation. Analytical pure ethanol was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai China) for conducting the ethanol precipitation process. Amino acids were purchased from Sigma-Aldrich, Co. (St Louis, MO, USA), and the rest reference reagents, including several saccharides and Danshen phenolic acids, were purchased from Shanghai Winherb Medical Technology Co., Ltd. (Shanghai China) for metabolite typical signal assignment. Ultrapure water was prepared using a Millipore Milli-Q System. Crude extract of Danshen as the raw material of the ethanol precipitation were provided by Guizhou Baite Pharmaceutical Co. Ltd (Guiyang, China).
2.2 General experimental procedures
2.2.1. Experiment operation
The detailed process of conducting the ethanol precipitation at laboratory scale was as follows:
The 8.0 g concentrated crude extract of Danshen with different solid concentration was placed into a 50 mL reaction reactor as the raw material for ethanol precipitation. During the process, a certain concentration and volume of ethanol-water solution was added into the tube with continuous and vigorous mechanical stirring. Then the mixture is refrigerated in a low-temperature thermostat bath for a period of time. After that, the solid-liquid mixture was filtered by a high - speed centrifuge. The obtained supernatant was the process product that we need to collect to perform further NMR analysis. All intermediate products of ethanol precipitation process were stored at -20 ℃ until analysis and would be thawed to room temperature for sample preparation.
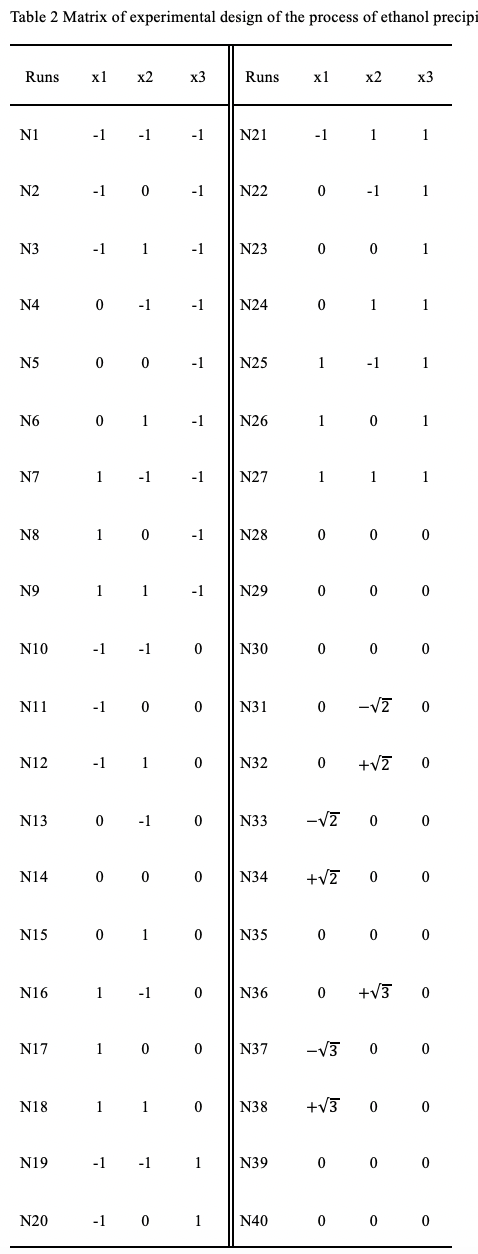
2.2.2. Experimental Design
To obtain reasonably distributed and representative enough samples, as well as to facilitate process statistical modeling, the experimental design was carried out in this study. The most significant variables affecting the process of ethanol precipitation were identified as ethanol-material ratio (EMR), apparent ethanol concentration (AEC) of the ethanol-material mixture and moisture concentration (MC) of the raw material 13–15. Then, a three-level full factor design with additional central composite circumscribed experiments were orchestrated, using the software MODDE (Version Pro 12, Umetrics, Umeå, Sweden).
The experimental matrix was designed with 7 repeated central points, and the variables were tested at three coded levels: ![]() 1, 0, and
1, 0, and ![]() 1, while the level of special “star points” in the design were
1, while the level of special “star points” in the design were ![]() ,
, ![]() ,
, ![]()
![]() .
.
The range and levels (coded and true) of variables are given in Table 1. All of the runs except the center points were performed in triplicate, and the mean values were used for analysis. The design matrix is shown in Table 2.
|
Variables to be studied |
Symbols |
Coded levels |
|||
|---|---|---|---|---|---|
|
Coded |
Truth Value |
-1 |
0 |
+ 1 |
|
|
AEC (%) |
x1 |
X1 |
65.00 |
72.50 |
80.00 |
|
EMR (g•g− 1) |
x2 |
X2 |
1.50 |
2.50 |
3.50 |
|
MC (g•g− 1) |
x3 |
X3 |
0.38 |
0.45 |
0.52 |
2.3. Sample Preparation
When sampling from each ethanol precipitation supernatant, weight of different sample was adjusted to a quantity equal to 0.08 g per gram dry matter in the raw material as a standard unit. In such a mass-normalized way, peak area of the same compound at the same chemical shift in different samples became quantitatively comparable, which can help with calculating the retention rate of compound by comparing the specific peak area.
To weaken the effects of residual water and/or ethanol in the solution, ethanol precipitation supernatant was weighed and dried using a centrifugal vacuum concentrator at 60 °C for 2 h. The dehydrated sample was added into exactly 540 µL of ultrapure water and 60 µL of D2O (containing 0.05% TSP). The mixture was dissolved by ultrasound for 3 minutes and centrifuged at 3 500 r/min for 10 min to remove insoluble substances. Then, the supernatant solution was transferred into a standard 5‑mm NMR tube for measurement.
2.4. 1H NMR analysis
2.4.1. 1H NMR analysis conditions
All 1H NMR spectra were recorded by a Bruker AVANCE III NMR spectrometer (Bruker, Karlsruhe, Germany) operating at 500.13 MHz at room temperature. The NMR probe was frequency tuned and impedance matched before each acquisition. Two 1H NMR pulse sequences applied in this study were as follows: a presaturation sequence PROTON to suppress the residual water signal and a standard NOESYPR1D pulse sequence to obtain 1H NMR spectra. They were both from Bruker pulse program library.
Preparative test was conduct to select acquisition and processing parameters of 1H NMR spectra in this work. Relevant parameters were adjusted to an appropriate combination, so as to obtain accurate and precise measurements. Each 1H NMR spectrum with 32 K data points required 6‑min acquisition time and employed following parameters: sampling time 2.980 s, pulse width 12 ppm, relaxation delay 2.0 s, 90° pulse width of 14.75 µs. Receiver gain value was at 57 and free induction decay (FID) was acquired 64 times for increasing signal-to-noise ratio (S/N) without causing baseline distortion. A 13C NMR experiment and a 2-dimensional (2D) 1H-13C heteronuclear single quantum coherence (HSQC) were acquired for the auxiliary reference to assist the identification of the constituents within the Danshen extracts and processed with similar parameters as described previously.
2.4.2 1H NMR data processing
Bruker Topspin (version 3.2.6, Bruker Biospin Corporation, Billerica, MA, USA) was used for data preprocessing. FIDs were weighted by an exponential function with a 0.3-Hz line broadening factor prior to Fourier Transformation. Phase corrections, baseline correction and displacement rectification were performed manually for ensuring a better evaluation of the signals. When carrying out the displacement rectification, the peak of TSP was setting at 0.00 ppm as internal reference to achieve unified peak alignment. The manual mode was applied also for the signal integration process choosing the same width for each compound.
MestReNova (version 14.0.0, Mestrelab Research, Santiago de Compostella. Spain) was used to segment (bin) every spectrum by 0.02 ppm of bucket width across the chemical shift δ 0.50–10.00 ppm. The signal intensity in each region were integrated by peak mode. The regions of δ 4.90–4.70 and δ 1.28–1.21 were excluded from the analysis to avoid the effects of the residual solvent (H2O and CH3CH2OH) resonance. Subsequently, because of the overlapping of proton signals, a deconvolution method was adopted to quantify the typical peak area of chemical markers in all the samples for more precise process interpretation, using linearly fitting coupled with the Lorentzian and Gaussian mixed function.
2.5. Chemometric analysis
2.5.1 Sample classification analyses
OPLS-DA was applied to explore differential metabolites between the samples collected before and after ethanol precipitation. After the alignment of all the NMR spectra according to the TSP typical signal at 0.00 ppm, the spectra region between δ = 0.50 and 10.00 ppm of process intermediates obtained before and after the ethanol precipitation were partitioned (“bucketed”) into 475 regions of 0.02 ppm. All the buckets were integrated by peak of intensities and scaled to total intensity in scaling process. Then the pretreated spectra were exported to the software SIMCA (Version 14.1, Umetrics, Umeå, Sweden) to conduct further chemometrics analysis. Then, Pareto scaling method, which uses the square root of standard deviation as the scaling factor 28, was used before two-class OPLS-DA in order to balance the importance of high value metabolites and low value metabolites while keeping the structure of the data partially intact. A permutation test was performed using 50 permutations to check overfitting of the OPLS-DA model and confirm whether the model was valid or not. Unsupervised principal component analysis (PCA) and corresponding hierarchical classification analysis (HCA) tree were applied to classify the designed samples and get an overview of differences or similarities between sample profiles and to detect possible outliers.
2.5.2 Multiple regression between process variables and chemical markers
The experimental results were analyzed using multiple regression technique in the software MODDE by MLR models, which would use typical features in the dataset to establish a relationship to known how the variables impact on the transfer and transformation of chemical markers, and the process efficiency 29.
Because of the mass-normalized sample preparation method mentioned in Sect. 2.3, every retention ratio of compound through a specific ethanol precipitation process, taken as the response of the MLR model, was calculated by Eq. (1):
![]() (1)
(1)
where ![]() and
and ![]() are the typical quantitative peak area of the compound in the NMR sample of the raw material and ethanol precipitation supernatant, respectively. A study on methodology of the method is shown in the Table S1.
are the typical quantitative peak area of the compound in the NMR sample of the raw material and ethanol precipitation supernatant, respectively. A study on methodology of the method is shown in the Table S1.
Every predicted retention ratio of chemical marker through a specific ethanol precipitation process fitted by the regression model is listed as the quadratic empirical equation (2):
![]() (2)
(2)
where ![]() is a constant,
is a constant, ![]() ,
, ![]() , and
, and ![]() are the linear, quadratic and interactive coefficients, respectively,
are the linear, quadratic and interactive coefficients, respectively, ![]() and
and ![]() are the coded values of the variables. Equations would be used to explain the classification rule displayed in the PCA model.
are the coded values of the variables. Equations would be used to explain the classification rule displayed in the PCA model.
All the statistical analysis was based on the confidence level of 95%.