Materials And Methods
This study complied with the Helsinki Declaration and approval from the Ethics Committee of our center was obtained. All patients provided their informed consents for the publication of their images/data.
Patient characteristics and CT simulation
Fifteen patients with cervical cancer undergoing radical hysterectomy and adjuvant pelvic radiotherapy in our hospital were chosen for this research between August 2019 and November 2019. The selection criteria were staged ⅠB-ⅡA, biopsy-proven squamous cell carcinoma. The 15 eligible patients ranged in age from 45 to 65, the mean and median age was 52.3 and 57 years old, respectively. The thermoplastic pelvic masks were used to immobilize the patients with supine position. All patients were scanned CT simulations of 5 mm slice thickness using a Philips 16-slice Brilliance big bore computed tomography scanner (Philips Medical Systems, Amsterdam, Netherlands), and with comfortably full bladder (after emptying, patients were requested for drinking 1 liter of water 30 to 45 minutes before treatment and holding urine) and a bowel preparation with oral contrast agent prior to simulation. The scan images were performed from the L2 vertebra to the area 5cm below the symphysis pubis.
Target and normal tissue volume definition
Contouring was performed on the platform of Monaco 5.11 (Elekta AB, Stockholm, Sweden) planning system. All outlines were represented by the same radiation oncologist for consistency. Delineation was according to the recommendations of Radiation Therapy Oncology Group (RTOG) 0418 protocol and the International Commission on Radiation Units and Measurements reports (ICRU) Report 62. The clinical target volume (CTV) included the vaginal stump, parametrial soft tissue and pelvic lymph drainage area. The CTV ranged from the L4-L5 vertebra to the inferior margin of the obturator foramen. The planning target volume (PTV) was generated by expanding a uniform 7mm margin from the CTV. The organs at risk (OARs) included small bowel, bladder, rectum, spinal cord and femoral heads.
Treatment planning
To ensure the consistency, all treatment plans were operated by the same radiation physicist. The FF-IMRT and VMAT plans were done with Monaco planning system version 5.11, and executed with Elekta Synergy Linac (Elekta Ltd., Crawley, UK) equipped with 8 MV photon beams and the MLCi2 (40 pairs MLC leaves and each one is 1 cm width at the isocenter). The prescribed dose of PTV was 1.8/45 Gy. The volume of PTV receiving >49.5Gy was limited to <1%. The volume of small bowel receiving >30 Gy was limited to <50%; <50% of the rectum was to receive >30 Gy, <35% of the bladder was to receive >40 Gy, and <5% of the femoral head was to receive >40 Gy. The maximum doses (Dmax) to the small bowel and rectum were both confined to lower than 48 Gy.
Some patients who had positive surgical margins also received brachytherapy using Ir192 source (high-dose rate) once a week, applied 18Gy/3fractions, dosed at the vaginal surface.
HT plans
The HT plans were operated using the tomotherapy planning station with 6 MV x-ray and were implemented on the Tomo HD (Accuray Inc., Madison, USA). Parameters for beamlet calculation were a field width of 2.5 cm, pitch values of 0.287, modulation factor of 3 and normal dose calculation grid.
VMAT plans
All VMAT plans were created using the Monaco 5.11 planning system and one beam with double 360° arcs were used, and there were 100 control points per arc. All VMAT plans were computed using Monte Carlo algorithm.
Fixed-field IMRT plans
Nine evenly distributed coplanar fields with the gantry angles of 200°, 240°, 280°, 320°, 0°, 40°, 80°, 120° and 160° were used, for each setting 20 control points. All FF-IMRT plans were computed using Monte Carlo algorithm. The optimization functions of the FF-IMRT plans were same to those of the VMAT plans. The DMLC (sliding window) technique was used in the FF-IMRT plans.
Plan evaluation parameters
The research analyzed the dose volume histograms (DVHs) obtained from the PTV and other contoured OARs. Dosimetric parameters were quantified from PTV including D98 (the dose received 98% volume of the PTV), D50, D2, the mean dose (Dmean), conformity index (CI) and homogeneity index (HI). CI was used to evaluate the conformity of prescribed dose distribution.
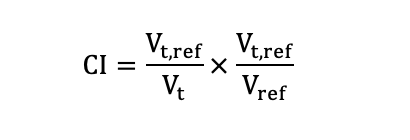
Here, Vt,ref, Vt and Vref denoted the target volume receiving the prescribed dose, the target volume and the total volume covered by the prescribed dose, respectively. The CI ranges from 0 to 1, with a higher CI indicating better conformal dose of the target. According to ICRU report NO.83[17], the HI was calculated as following formula:
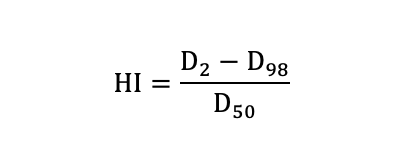
HI was used to indicate the uniformity of dose distribution. The smaller value of HI means a better homogeneity of the target volume.
For the OARs (small bowel, rectum, bladder and femoral heads), data analysis was carried out for the V5 (the OAR volume received the dose of 5 Gy), V10, V20, V30, V40, Dmean and Dmax. Treatment delivery time of each plan was also collected and compared.
Data analysis
The data collected from DVH of different plans were compared with paired t-test using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA). All P values were two-sided, and a P value < 0.05 was considered statistically significant.