Methods
Study design
The present study is a randomized clinical trial controlled with placebo and two-blinded groups that conform to standard protocol items: recommendations for interventional trials statement (SPIRIT) 2013. The study will be conducted at Imam Reza Hospital of Tabriz, Iran.
Participants
In this study, the target population is patients with diabetes mellitus undergoing hemodialysis. The inclusion criteria are: an age³ 20 years; body mass index=18.5-30 kg/m2; having type 2 diabetes mellitus; three times per week hemodialysis; being on hemodialysis for at least 6 months; ability and willingness to cooperate in the study. The exclusion criteria are having inflammatory or infectious diseases; receiving steroidal or nonsteroidal anti-inflammatory drugs, smoking, and using NS oil regularly.
Interventions
The patients are randomly allocated to either a NS oil or control group. Patients in the NS oil group will receive two soft gel of NS oil, whereas the control group will receive two soft gel of paraffin oil (each soft gel weights 1 gram). Treatment of both groups will last for 12 weeks. The selected dose is based on Kaatabi H(11), which is one of the best effective and safe interventions of NS oil supplementation in patients with diabetes mellitus. According to the predominant studies, NS oil supplementation in the mentioned dose and the term, but even in more prolonged use, did not have certain complications. just in some rare cases, mild and temporary nausea and dyspepsia and decreased appetite have been reported(12, 13, 22)Both soft gels will be prepared by the pharmaceutical company in a completely similar way in terms of shape, color, and odor. The preparation of black seed oil will be done by the cold press method by the pharmaceutical specialist.
The supplements will be available to attendees every two weeks. All patients will be asked to their usual dietary habits, physical activities, and drug regimens and report us any possible changes. In addition, every two weeks, all patients will be evaluated by interview. Furthermore, the researcher will give participants a call number to inform him if there are any side effects or other problems.
Outcomes measures
Accordance with the inclusion criteria, after explaining the objectives and method of the study, patients will be consciously entered into the study. They will be asked to obtain Informed consent. Next to that, demographic and anthropometric indices checklist for each individual are filled, and general nutritional recommendations will be taught by a nutritionist. Dietary intake of individuals will be evaluated by the 3-day food registration questionnaire (two-week days and one weekend day). Also, before the intervention, patients will be paid to complete their standard questionnaires, including KD-QOL and HADS, to assess the quality of life, depression, and anxiety. If the patient is illiterate, the information will be received by interviewing. At baseline and the end of the 12th week, 10 mL of blood will be obtained from each patient after 12 hours of fasting. Blood serum will be stored at refrigerator temperature (-70°C). Laboratory tests including renal function tests as creatinine, urea, uric acid, urine volume, indices related to blood glucose including FBS, HbA1c, GA, serum insulin, insulin resistance and β-cell function, indices related to oxidative stress and inflammation including SOD, MDA, TAC, and hs-CRP will be measured and recorded.
The process for assessing the activity of hs-CRP, MDA, SOD, and TAC will be done by Navand assay kit (Navandsalamat, Iran) according to the company package insert instruction. The serum insulin level, HbA1c, and GA will be measured by enzyme-linked immunosorbent assay (ELISA) method. Biochemistry Solutions will also measure creatinine, urea, uric acid, and FBS.
In order to determining insulin resistance and β cell function, HOMA-IR and HOMA-B formula will be used respectively(23).

The reliability of laboratory methods by sending the first ten samples of experiments with two different names to the laboratory expert and investigating the level of agreement between them will be determined. Also, body composition will be evaluated by the bioelectrical impedance analysis (BIA) method.
Sample size
In this study, by considering α = 0.05, and the power of 90% and using primary data on creatinine outcome, based on findings of Z. Ansari, SJKDT, 2017 study (1) based on the procedure of two independent groups G-power software or Formula
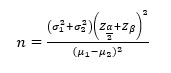
The minimum sample size required for each group is 20 subjects, which with predicting 10% loss it will be 23 patients in each group.
Stratified randomization
The patients will be allocated to either a NS oil or control group by block randomization after stratification based on the frequency of hemodialysis per week (2 or 3 times per week) and the amount of blood sugar (FBS< 120 mg/dL, FBS= 120-200 mg/dL and FBS> 200 mg/dL). This process will be carried out by a statistics specialist using of RAS (random allocation software) in block sizes of 4. A trained dietician will perform blinding, and the patients and researchers will be kept blinded to the allocation.
|
Table. 1 Timetable of planned activities during the study directly related to participants |
|||||
|
|
Study period |
||||
|
Enrolment |
Allocation |
Post allocation |
Follow-up |
||
|
Time Point |
Weeks -4 to -1 |
Week -1 to 0 |
Week 0 |
Week 12 |
Week 13 |
|
Enrolment: Eligibility screen Informed consent Allocation |
* |
|
|
|
|
|
* |
|
|
|
|
|
|
|
* |
|
|
|
|
|
Intervention: Two placeboes soft gel daily Two nigella sativa soft gel daily |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assessments: 24 h food recall Blood collection Urine collection Anthropometric measurements KDQOL questionnaire HADS questionnaire |
|
* |
|
|
* |
|
|
* |
|
|
* |
|
|
|
* |
|
|
* |
|
|
|
* |
|
|
* |
|
|
|
* |
|
|
* |
|
|
|
* |
|
|
* |
|
KDQOL: Kidney Disease Quality of Life; HADS: Hospital Anxiety; and Depression Scale
Statistical analysis
The collected data will be analyzed by the statistical package for the social sciences (SPSS) version 23 software. Descriptive statistics, including frequency, percentage, and central indices and dispersion will be done. The Wilcoxon signed-rank test will determine the normal distribution of the data. If data distribution is normal, to compare the serum markers and physical components analysis among the study groups in the pre-intervention stage, independent sample t-test, and after the intervention, analysis of covariance (ANOVA) will be used by modulating the baseline values and probable variables. Paired-samples t-test will be used to compare data in each study group. If the data distribution is normal, for comparison of the serum markers and physical components analysis before the study, Mann-Whitney test and among the study groups, Wilcoxon signed-rank test will be used (for both before and after the intervention). In all tests, the p-value< 0.05 will be considered significant. missing data will be removed from the final analysis.
Ethics considerations
The expert nutritionist will explain the purpose of the study, benefits, or side effects of the supplements to all patients before obtaining written informed consent. The volunteers will fill an informed consent form and enrolled in the study. They can withdraw their consent at any time study.
According to previous studies, this supplement's use is safe in the dose, as mentioned earlier, and has no side effects. In case of any acute clinical symptoms due to supplementation, the subjects will be excluded, and the researcher will pay the costs of any possible complication. Any adverse events and other unintended effects of trial interventions will be collected and mentioned in the article.
The personal information of enrolled participants will be collected, shared, and confidentiality maintained before, during, and after the study by encoding systems. The results of the experiments will be available for participants at the end. The participants will be assured that the project will not be charged for treatment interventions. If NS supplementation is effective, the control group will also take this supplement.
The benefits of participating in this study for control group, are free:
- Nutritional counseling and monthly monitoring
- Glycemic tests at the beginning and end of the study
- Kidney function tests at the beginning and end of the study
- Inflammation and oxidative stress status tests at the beginning and end of the study
- Body composition and anthropometric tests at the beginning and end of the study
All results will be delivered to the participants at the end of the study.