Methods
Materials: Cocoons were purchased from the Shandong Institute of Sericulture. Gentamicin was purchased from the National Institutes for Food and Drug Control. Protease Type XIV from Streptomyces griseus was purchased from Sigma-Aldrich (St. Louis, MO, USA), and other inorganic analytical reagents were purchased from Shanghai Aladdin.
Preparation of internal fixation materials: A gentamicin-loaded silk-based material was prepared by a previously reported method8,9. The cocoon was boiled in 0.02 mol/L sodium carbonate solution for 30 min, washed thoroughly with distilled water three times and placed in an electro-thermostatic blast oven at 60°C for at least 12 h to yield refined silk. A mass of 10 g of the refined silk was added to a ternary solution of CaCl2•CH3CH2OH•H2O (in a 1:2:8 molar ratio). The solution was heated to maintain the temperature at 85°C until the silk dissolved completely. The solution of the dissolved silk fibroin was placed in a dialysis bag (MWCO 3,500, Pierce) and dialyzed with deionized (DI) water at 4°C for 2 d to yield a pure silk fibroin solution. The DI water was changed hourly during the dialysis. The silk fibroin solution was centrifuged twice (18,000 rpm; 20 min/time) in a high-speed low-temperature centrifuge (4°C). The supernatant was collected and frozen at -20°C for 5 d and dried in a vacuum freeze dryer (-79°C). Gentamicin sulfate was dissolved in hexafluoroisopropanol (HFIP) at four concentrations (40, 20, 15, and 10 mg/ml), and pure HFIP was also prepared. The vacuum-dried silk (10 g) was cut into pieces and placed in a 50-ml syringe. A volume of 40 ml of the prepared HFIP solution was added to the syringe, and complete dissolution of the silk fibroin at room temperature yielded a 25 w/v% silk fibroin/HFIP solution. The solution was poured into a rod-shaped mold made of paraffin (a hollow column with a height of 33 mm and an inner diameter of 8 mm). The mold was completely immersed in a methanol solution for 3–4 d. A methanol-to-water gradient was carried out four times at 1-h intervals to gradually replace 100% methanol with 100% water. The rod-like material was left immersed in the water for an additional 2 d and then removed and dried in an oven at 60°C. Rods with a height of approximately 30 mm and a diameter of approximately 5 mm were thus obtained. Some of the rods were processed using a professional lathe to fabricate screws. Four gentamicin-loaded silk-based antibacterial materials (with gentamicin concentrations of 16 mg/g (GSS1), 8 mg/g (GSS2), 6 mg/g (GSS3), and 4 mg/g (GSS4)) and a pure silk-based material (PSS) were thus obtained.
In vitro degradation: Five rod-like samples (approximately 5 mm in diameter and 15 mm in height) were completely dried and weighed (the weight is denoted as m0). The samples were placed in a six-well plate (one sample per well), and 5 ml of a 1 mg/ml protease XIV solution were added to each well to completely immerse the samples (n = 3). At 37°C, the soaking solution was discarded every 48 h, the sample was washed with phosphate-buffered saline (PBS), and fresh protease XIV solution was added to the sample. At 7, 14, 28, 56, and 84 d, one sample was removed, washed thoroughly with PBS, dried thoroughly, and weighed (the weight was denoted as m1). The degradation rate (%) was calculated as 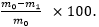
In vitro water absorption: The diameters and weights of five completely dried rod-like samples (approximately 5 mm in diameter and approximately 15 mm in height) were obtained (n = 3) and denoted by Wd and Dd, respectively. One rod-like sample was placed in an Eppendorf (EP) tube, 3 ml of PBS were added to immerse the material, and the tube was maintained at 37°C. Samples were withdrawn at predetermined time points (10 min, 20 min, 30 min, 1 h, 2 h, 6 h, 12 h, 24 h, 48 h, 72 h, 96 h, 120 h, and 144 h), filter paper was immediately used to absorb moisture on the sample surface, and the sample diameters and weights were measured and denoted by Ws and Ds, respectively. The water absorption and expansion rates were calculated using the following formulas: water absorption rate 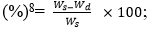 expansion rate (%) =
expansion rate (%) =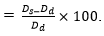
Animal experiments: Six New Zealand rabbits were numbered, weighed, and injected with 3% pentobarbital for anesthetization. The skin of each rabbit was prepared by removing the hair on both hind legs. The skin of one hind leg was cut open, and a retractor was used to pull the muscles away to expose the femoral condyle. An electric drill was used to drill and tap the femoral condyle from outside to inside, the GSS4 antibacterial silk-based screw (with a tail diameter of 5 mm, a body diameter of 4 mm and a length of 10 mm) was implanted, and the wound was sutured. The other hind leg of the rabbit was treated the same way. Two New Zealand rabbits were killed at 4, 8, and 12 weeks after the operation, and the four lower limbs were scanned by microcomputed tomography (Micro-CT) at each time point. Finally, the femur was dissected to observe the screw and surrounding tissue conditions. Micro-CT was used to measure the body diameter (d) (n = 4) of the screw in the femur at different time points, and the rate of change in the diameter (%) was calculated as 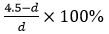 (the body diameter of the screw of 4.5 mm did not change after being soaked in a PBS solution for 48 h).
(the body diameter of the screw of 4.5 mm did not change after being soaked in a PBS solution for 48 h).
All experimental protocols were approved by Foshan First People's Hospital licensing committee and all methods were carried out in accordance with relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines.
Statistical analysis: All the experimental data are expressed as the mean ± standard deviation, based on the results for at least three experimental samples. SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. The one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) tests were performed for comparative analyses among multiple groups, and Dunnett’s T3 was used to compare the heterogeneity of variance. The significance level of the test was defined as α = 0.05, and differences were considered statistically significant at p < 0.05.