Methods
Study design
The present study was a randomized, double-blind, sham-controlled research study. Participants were randomly assigned to the active and sham groups. PA training simultaneously with left anodal/right cathodal tDCS was given to the active group participants, on the other hand participants in sham group had sham tDCS with PA intervention.
Participants
Study population consisted of 28 students with DD. There were 14 participants in the active group (12 males, 2 female) 7-11.08 years old (mean age 9.18, SD = 1.30 years). The sham group had 14 participants (10 males, 4 females) 7.08-11.10 years old (mean age 9.54, SD = 1.15 years). Dyslexia diagnosis was made based on the DSM-5 criteria for reading disorder (59); a speech-language pathologist assessed the type of dyslexia using a set of diagnostic tests, including word and non-word (NW) subtests from the Nema reading and dyslexia test (60) to include those with phonological dyslexia. Internal consistency for word and NW reading subtests was 0.098 and 0.095, respectively (61). Dyslexic students were collected from primary schools and learning disability centers and evaluations were performed in a private speech therapy clinic. Inclusion criteria included having correctness and/or speed of word and/or NW reading subtests at least 1.5 standard deviations under the population mean for the educational grade (60), a non-verbal IQ score>85 (62), not having associated disorders including seizure, attention deficit and hyperactivity disorders evaluated by clinical observation and by using Conners’ Rating Scales—Revised (63), non-compensated hearing loss, native Persian speaking and being right-handed as evaluated by the Edinburgh inventory (64).
Exclusion criteria included history of a reading related intervention before treatment and use of prescribed medication associated with the cranial neural system disorders. An informed consent form, describing the aim of the study and processes involved was signed by the parents of all students after.
Interventions
Intervention sessions (for both groups) were performed at the rehabilitation clinic in Tehran, Iran. Regardless of group allocation, each participant went through 15 60-minute intervention sessions [three times per week (T) for 5 weeks] (65). The study outcomes were measured at five steps: immediately before treatment (baseline) (T0) , at the end of the fifth intervention sessions (T1), at the end of the tenth intervention session (T2), immediately after the intervention (T3), and 6 weeks after the end of the intervention (T4) by a blinded investigator. To evaluate the stability of the treatment in both groups, 6-weeks follow-up was considered for each participant.
PA Intervention
PA treatment was performed in both the active and sham groups each session. The duration of each treatment session was 60 minutes (15 hours in total). We derived the PA intervention program from the ‘‘Gillon Phonological Awareness Training Program’’ (66). The Gillon PA treatment program covers nine skills(66):
- Rime abilities
- Phoneme analysis
- Phoneme recognition
- Phoneme segmentation
- Phoneme blending
- Tracing speech sounds
- Sound–symbol association
- Tracing speech sound with letters
- Reading and writing skills
According to the Gillon PA program, the time spent on each skill varied based on the participant’s increasing skill level. For example, rhyme and phoneme analysis and recognition which are considered initial tasks were addressed in the first 4 to 6 sessions. Toward the end of the program (after 6 or 7 sessions), phoneme segmentation, blending and manipulation were performed and reading and writing skills were trained. Participant did not need to succeed completely with one before moving on to the next skill. Based on the progress of individuals in developing learning skills, other skills could be added in one session (66).
tDCS
Participants were randomly assigned to the sham and anodal tDCS. In order to determine the location of electrodes accurately, we used an EEG cap. The anode and the cathode electrodes were positioned over the left and the right parieto-temporal regions, respectively. An Activa Dose II Iontophoresis Delivery Unit tDCS was used to transit tDCS. In the active group, stimulation was performed by transiting the direct current between two conductive rubber electrodes. Each electrode was covered in a sponge pad. Two 5 cm x 7 cm electrodes were positioned on the scalp using an elastic rubber strip (Figure 1). Both sides of the sponge pads were moistened in dextrose 3.33% and sodium chloride 0.3% solution for better conductivity. Prior to placing the electrodes on the scalp, the therapist checked the head skin for any skin lesions. Electrodes position were similar in both the anodal and sham groups. Location of stimulation in the left and the right hemispheres coincided with the midway between T3 and P3 and midway between T4 and P4 according to the 10-20 international system (67). An anodal and a cathodal stimulation augmented the left parieto-temporal function and reduced the right parieto-temporal function, respectively. The bilateral stimulation motivates normalization of atypical brain function detected in dyslexic participants in the phonological tasks (50). In the active group, at the beginning of stimulation, the current was gradually increased over the first 30 seconds to 1 mA, the excitation threshold, as ramp-up and was declined gradually to 0 mA over the last 30 seconds as ramp-down at the termination of the stimulation. In the interval between the ramp-up and ramp-down, a steady direct current of 1 mA was transited for 20 minutes. In the sham group, the direct current was given to the brain only for 30 seconds and was turned off afterwards. This placebo-intervention induces tDCS-induced sensation (e.g. irritation and itching) indiscernible by the participants from an active intervention (68). Intensive combined intervention was applied three times a week for 5 weeks (15 sessions). tDCS was applied to the active group for 10 sessions with PA intervention, simultaneously (During the 60 minutes of the combined intervention, only 20 minutes of stimulation was provided to the active group participants).
Safety
Students filled in a questionnaire on adverse effects at the end of each treatment session. The items in the questionnaire were: "headache, neck pain, scalp pain, tingling, itching, burning sensation, skin redness, sleepiness, trouble concentrating and acute mood change". Participants gave each item a score from 1 to 4 (1, absent; 2, mild; 3, moderate; 4, severe). Students scored the intensity of their adverse effects (69). Turning off and resetting the device took place in case of moderate to high rated for adverse effects. If side effects remained, the stimulation intervention was postponed until the next session.
Outcome Measures
In the present study, RAN and VSTM abilities were evaluated using researcher-developed RAN task and VSTM related sub-tests from Wechsler Intelligence Scale for Children—Revised in Iran(62), respectively.
RAN task: In this study, a RAN task was designed based on Denckla (8). The sub-tasks of the RAN task are as follows:
- RAN_ objects: objects selection was based on the frequency of occurrence in Persian, more familiar semantic concepts for the student, mono-syllabic structure and simple production structure. For this purpose, Persian core vocabulary study was used(70) which has extracted all perceptual and productive words of students from first to fifth grade. Therefore, all the vocabulary produced by primary school students were examined and a list of words that met the above criteria was constructed (ball, table, bed, dish and hand).
- RAN_ colors: colors were selected based on the frequency of occurrence (high-frequent) and simple syllabic structure from Persian colors category(71). These words were examined and 5 colors that had the highest frequency in elementary school textbooks (blue, green, black, red and yellow) were selected.
- RAN_ digits: mono-syllabic digits were selected based on their frequency of occurrence in elementary school math textbooks (1, 2, 3, 6, 9).
- RAN_ animals: The most frequent and well-familiar animals in Persian, in accordance with the words of the textbook of primary schools were selected(70). The animal names ranged from mono-syllabic to tri-syllabic (cow, dog, mouse, horse, pigeon).
- RAN_ high-frequency capital letters: These letters were selected based on Mansouri high-frequency capital letters performed in Persian (آ، ت، م، ن، ب) (72).
- RAN_ high-frequency lowercase letters: These letters were selected based on Mansouri high-frequency lowercase letters performed in Persian (ا، نـ ، تـ ، لـ ، مـ ) (72).
- RAN_ low-frequency capital letters: These letters were selected based on Mansouri low-frequency capital letters performed in Persian (غ، ط، ض، ث، ک) (72).
- RAN_ low-frequency lowercase letters: These letters were selected based on Mansouri low-frequency lowercase letters performed in Persian (چـ ، صـ ، غـ ، (کـ ، ضـ (72).
Photographs were extracted from the Stanford-Binet IQ test_ Persian version in pictured sub-tasks (RAN_ animals and, RAN_ objects)(73).
Each of the five items in RAN sub-tasks was illustrated by color photographs organized randomly in 10 columns × 5 rows on a white A3 sheet. The letters and digits typed with B Nazanin size 36. Each sheet was placed on the table in front of the child, orderly. Initially, task instructions were then explained to the participant and the task items were preceded by examples items which involved a collection of addition items to prevent pre-activation of the task real items. Participants had to name all the items from right to left rapidly and correctly. It is noteworthy that if needed the meaning of the words were explained in case the child wasn’t aware of it. Timing started with the participant's first naming after "Go" using turning on a stopwatch. After naming all the items in each sub-task, the stopwatch was turned off. Total naming time for each sheet/sub- task and number of errors was noted on a separate sheet. Self-corrections were not considered as an error but time was consumed. RAN time was calculated in seconds. There was a 1 minute break between each sub-task naming.
VSTM Test: In the present study, to evaluate VSTM were used from forward digit span (FDS) and backward digit span (BDS) and non-word repetition test (NWRT). These subtests are as follows:
- FDS and BDS: to evaluate these abilities, the FDS and BDS subtests from Wechsler Intelligence Scale for Children - Persian version- were used(62); each span (FDS and BDS) including 2 sets; each of them with 7 series of digit (total digits=14) for repetition in forward and backward order. Digits in each series increase gradually (3-9 digits in FDS and, 2-8 digits in BDS). For correct repetition of each series, a score is awarded to the participant. If a participant fails to repeat each digits series, no score will be awarded. The maximum score for each span is 14. The test re-test reliability of the sub-tests of this test were 0.44 to 0.94.
- The NWRT: in this study NWRT was used to evaluate NWR ability (74). This test consists of 40 non-words (NWs) (1-3 syllables) that were created by changing one or two phonemes of the real word phonemes. NWs do not convey meaning and are not available in the Persian vocabulary but are in accordance with the rules of Persian phonology. In the process, items were presented with a live voice and a covered mouth. The time interval between the presentation of each NW was 3 seconds. All participants were given directions that they would have to repeat a non-sense item that with no concept. Participants were asked to express it as accurately as possible. Before the start of the test, 4 training items were presented to each participant. Participants were given the main target NWs to repeat. In case of completely correct repetition of each NW, a score of 1 and otherwise a score of zero was given to the participant. The maximum test score was 40 at this test. The content validity of the test was 95.5. Correlation coefficient between results of the NWRT, the FDS and BDS were 0.76 and 0.75, respectively (concurrent validity).
Outcome measures were evaluated at all five time points: T0, T1, T2, T3, and T4.
Randomization
To prevent collection bias and imbalanced confounding between the project groups, a computer-based randomization approach was used (www.sealedenvelope.com). To create a random archive, we included 28 participants and 4 blocks of the same size. To conceal the randomization process, a unique code, generated by the software, was given to participants. These unique codes were used on the cubes that represented the type of empirical groups and thus participants were randomly assigned into one of these groups.
Blinding
None of the participants, main therapist and evaluators were informed of group allocations. A sealed opaque envelope approach was used to conceal groupings. Assistant therapist received randomly created intervention assignments within sealed opaque envelopes. After a participant entered the study, the envelope was unsealed. Both the subjects and the main therapist who performed the intervention in both groups were blinded to the stimulation situation. Only the assistant therapist who set up the tDCS device was informed of the intervention allocation (active or sham). Additionally, the investigator involved in measuring outcomes and analysis was also blinded to group allocation.
Sample Size and Statistical Analysis
Sample size was calculated based on Cohen's scale (75) and using the G*Power software to compare the means for 5 measurements (76). Effect size was determined as f = 0.45, and the α and β errors were considered to be 0.05 and 0.20, respectively. A sample size of 14 participants in each group (28 participants in total) was determined.
Characteristics of the study population were presented as mean (SD, range) for continuous variables and frequency (%) for categorized variables (Table 1). Kolmogorov–Smirnov test was used to investigate the distribution of the data. Comparison of the two groups was performed using Student t-test or Mann Whitney U test for continuous variables and Chi square test or Fisher's exact test for categorized variables. Data of the two groups over time was analyzed using the repeated-measures analysis of variance (ANOVA) with the between-subjects factor group and within-subjects factor time. In all analyses, p < 0.05 was considered statistically significant. If the results of the ANOVA test showed significant time × group interactions, exploratory post-hoc Student's t-tests were used to examine significant differences at each time point between the two groups. To calculate the effect size, partial eta squared 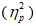 was determined. A Bonferroni correction was considered for all the performed tests. All statistical analyses were performed using the SPSS software version 23.
was determined. A Bonferroni correction was considered for all the performed tests. All statistical analyses were performed using the SPSS software version 23.