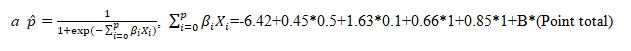Results
Patient characteristics
A total of 735 patients met the inclusion criteria. Patients with pineal cyst (n = 34), Rathke’s cleft cyst (n = 33), Mecune Alblight syndrome (n = 12), congenital adrenal hyperplasia (n = 10), and ovarian cyst (n = 19) were excluded. Finally, 627 patients were included and randomly separated into the training sample (n = 314) and validation sample (n = 313) (Fig. 1).
The mean age of the participants was 7.5 years [95% confidence interval (CI), 7.4–7.7 years]. The average disease duration was 1.0 years (95% CI, 1.0-1.2 years). CPP was diagnosed in 54.8% (172/314) and 55.0% (172/313) of patients in the training and validation sample respectively. Patients did not show significant difference of clinical and pelvic ultrasonography characteristics in the training and validation sample with an exception of the proportion of patients with a family history of CPP. Detailed description was showed in Table 1.
|
|
Training (n=314) |
Validation (n=313) |
P Value |
|
Central precocious puberty (%) |
172 (54.8) |
172 (55.0) |
0.9649 |
|
Clinical characteristics |
|
|
|
|
Age at onset of puberty [mean (SD), year] |
6.5 (1.6) |
6.5 (1.6) |
0.8132 |
|
Chronological age [mean (SD), year] |
7.5 (1.6) |
7.6 (1.7) |
0.9460 |
|
Bone age [mean (SD), year] |
9.6 (7.5) |
8.9 (2.3) |
0.1415 |
|
Bone age/ Chronological age (SD) |
1.2 (0.2) |
1.2 (0.2) |
0.8146 |
|
Duration of disease [mean (SD), year] |
1.1 (0.9) |
1.0 (0.8) |
0.7547 |
|
Family history of CPP (%) |
5 (1.6) |
0 (0.0) |
0.0250 |
|
Tanner stage for breast development |
|
|
|
|
Left (%) |
|
|
0.5959 a |
|
I |
9 (2.9) |
9 (2.9) |
|
|
II |
200 (64.5) |
211 (68.5) |
|
|
III |
98 (31.6) |
87 (28.3) |
|
|
IV |
3 (1.0) |
1 (0.3) |
|
|
Right (%) |
|
|
0.4052 a |
|
I |
12 (3.9) |
8 (2.6) |
|
|
II |
196 (63.2) |
211 (68.5) |
|
|
III |
99 (31.9) |
88 (28.6) |
|
|
IV |
3 (1.0) |
1 (0.3) |
|
|
Tanner stage for pubic hair development (%) |
|
|
0.1860 a |
|
I |
277 (88.2) |
282 (90.1) |
|
|
II |
32 (10.2) |
31 (9.9) |
|
|
III |
4 (1.3) |
0 (0.0) |
|
|
IV |
1 (0.3) |
0 (0.0) |
|
|
Height [mean (SD), m] |
130.0 (11.6) |
129.9 (12.6) |
0.8986 |
|
Weight [mean (SD), kg] |
28.4 (6.8) |
28.8 (7.3) |
0.5405 |
|
BMI [mean (SD), kg/m2] |
16.7 (2.2) |
16.9 (2.4) |
0.3691 |
|
LH [Median (IRQ), IU/L] |
|
|
|
|
Baseline |
0.43 (0.17, 1.07) |
0.43 (0.18, 1.02) |
0.6364 |
|
Stimulated |
10.45 (5.01, 21.36) |
9.55 (4.80, 24.97) |
0.9503 |
|
FSH [mean (SD), IU/L] |
|
|
|
|
Baseline |
3.7 (2.3) |
3.6 (2.2) |
0.6839 |
|
Stimulated |
16.7 (8.0) |
17.0 (7.8) |
0.6182 |
|
LH/FSH [Median(IRQ)] |
|
|
|
|
Baseline |
0.14 (0.08, 0.29) |
0.14 (0.07, 0.30) |
0.5607 |
|
Stimulated |
0.75 (0.38, 1.46) |
0.72 (0.32, 1.45) |
0.7394 |
|
Estradiol [Median (IRQ), pg/mL] |
15.0 (8.0, 29.5) |
14.0 (7.0, 26.8) |
0.4737 |
|
HCG [Median (IRQ), IU/L] |
0.08 (0.00, 0.23) |
0.05 (0.00, 0.21) |
0.4128 |
|
Prolactin [mean (SD), ng/mL] |
10.7 (7.7) |
9.7 (5.8) |
0.1042 |
|
DHS [mean (SD), μg/dL] |
53.3 (38.3) |
53.5 (39.6) |
0.9594 |
|
Testosterone [Median (IRQ), ng/dL] |
2.4 (0.0, 16.3) |
0.0 (0.0, 15.1) |
0.3793 |
|
Cortisol [mean (SD), μg/dL] |
8.3 (4.9) |
8.3 (4.7) |
0.9896 |
|
ACTH [Median (IRQ), pg/mL] |
24.2 (17.8, 33.0) |
24.5 (18.0, 34.0) |
0.7214 |
|
Total triiodothyronine [mean (SD), ng/dL] |
139.8 (24.3) |
136.5 (25.1) |
0.0982 |
|
Free triiodothyronine [mean (SD), pg/mL] |
3.9 (0.6) |
3.8 (0.6) |
0.8073 |
|
Total thyroxine [mean (SD), μg/dL] |
9.0 (1.8) |
9.1 (1.8) |
0.3588 |
|
Free thyroxine [mean (SD), ng/dL] |
1.0 (0.2) |
1.0 (0.2) |
0.6164 |
|
TSH [mean (SD), μIU/mL] |
2.4 (1.4) |
2.2 (1.2) |
0.1302 |
|
Pelvic sonogram |
|
|
|
|
Ovarian volume |
|
|
|
|
Average ovarian (%) |
1.9 (0.9) |
1.9 (0.9) |
0.6001 |
|
<1mL |
40 (12.7) |
44 (14.1) |
0.8866 |
|
1-<2mL |
157 (50.0) |
155 (49.5) |
|
|
≥2mL |
117 (37.3) |
114 (36.4) |
|
|
Largest ovarian (%) |
|
|
|
|
<1mL |
31 (9.9) |
34 (10.9) |
0.8914 |
|
1-<2mL |
142 (45.2) |
137 (43.8) |
|
|
≥2mL |
141 (44.9) |
142 (45.4) |
|
|
Smallest ovarian (%) |
|
|
|
|
<1mL |
67 (21.3) |
73 (23.3) |
0.8138 |
|
1-<2mL |
160 (51.0) |
153 (48.9) |
|
|
≥2mL |
87 (27.7) |
87 (27.8) |
|
|
Uterine |
|
|
|
|
Length |
|
|
|
|
<3cm |
278 (88.5) |
287 (91.7) |
0.1774 a |
|
3-<4cm |
36 (11.5) |
25 (8.0) |
|
|
≥ 4cm |
0 (0.0) |
1 (0.3) |
|
|
Volume |
|
|
|
|
<3mL |
244 (77.7) |
251 (80.2) |
0.7287 |
|
3-<4mL |
42 (13.4) |
36 (11.5) |
|
|
≥4mL |
28 (8.9) |
26 (8.3) |
|
|
Endometrium visible (%) |
40 (12.7) |
40 (12.8) |
0.9878 |
|
HCG: human chorionic gonadotropin; DHS: dehydroepiandrosterone sulfate; ACTH: adrenocorticotropic hormone; TSH: thyroid - stimulating hormone. a Calculated using Fisher’s exact test |
|||
Training sample
The crude relationship between potential predictors and the diagnosis of CPP was showed in Additional file 1 (Additional Table 1). A total of 21 variables with a P value of less than 0.20 were entered into the multiple variable logistic regression model. After a forward stepwise selection, a final model including four predictors (age at onset of puberty, basal LH, largest ovarian volume, and uterine volume) was selected (Additional file 1 [Additional Table 2]). The variance inflation factor was less than 2.0 for all predictors, indicating there was no linear relationship between predictors. The C-index was 0.86 [95% CI, 0.82–0.90; Fig. 2]. Hosmer-Lemeshow test demonstrated goodness of fit for the prediction model (P = 0.49). The calibration plot showed an intercept of -0.01, and a slope of 1.01 (Additional file 1 [Additional Fig. 1]). A bootstrap analysis (resampling the model 300 times) showed a corrected C-index of 0.86.
|
Predictors |
Categories |
Reference value (Wij) |
βi |
βi (Wij-WiREF) |
Pointsij = βi (Wij-WiREF)/B a |
|---|---|---|---|---|---|
|
Age at onset of puberty (years) |
< 1 |
0.5 = W1REF |
0.45 |
0 |
0 |
|
1 - < 2 |
1.5 |
0.45 |
1 |
||
|
2 - < 3 |
2.5 |
0.90 |
3 |
||
|
3 - < 4 |
3.5 |
1.35 |
4 |
||
|
4 - < 5 |
4.5 |
1.80 |
6 |
||
|
5 - < 6 |
5.5 |
2.25 |
7 |
||
|
6 - < 7 |
6.5 |
2.70 |
8 |
||
|
7 - < 8 |
7.5 |
3.15 |
10 |
||
|
Basal LH (IU/L) |
< 0.2 |
0.1 = W2REF |
1.63 |
0 |
0 |
|
0.2 - < 0.4 |
0.3 |
0.326 |
1 |
||
|
0.4 - < 0.6 |
0.5 |
0.652 |
2 |
||
|
0.6 - < 0.8 |
0.7 |
0.978 |
3 |
||
|
0.8 - < 1.0 |
0.9 |
1.304 |
4 |
||
|
1.0 - < 1.2 |
1.1 |
1.630 |
5 |
||
|
1.2 - < 1.4 |
1.3 |
1.956 |
6 |
||
|
1.4 - < 1.6 |
1.5 |
2.282 |
7 |
||
|
1.6 - < 1.8 |
1.7 |
2.608 |
8 |
||
|
1.8 - < 2.0 |
1.9 |
2.934 |
9 |
||
|
2.0 - < 2.2 |
2.1 |
3.260 |
10 |
||
|
2.2 - < 2.4 |
2.3 |
3.586 |
11 |
||
|
2.4 - < 2.6 |
2.5 |
3.912 |
12 |
||
|
≥ 2.6 |
3.0 |
4.727 |
14 |
||
|
Largest ovarian volume |
1 |
1 = W3REF |
0.66 |
0 |
0 |
|
2 |
2 |
0.66 |
2 |
||
|
3 |
3 |
1.32 |
4 |
||
|
Uterine volume |
1 |
1 = W4REF |
0.85 |
0 |
0 |
|
2 |
2 |
0.85 |
3 |
||
|
3 |
3 |
1.70 |
5 |
||
| a We define the constant B for the points system (the number of regression units that will correspond to one point) as the increase in risk of CPP associated with a 0.2 (IU/L) increase in basal LH: B = 0.2*1.63 = 0.326 | |||||
| Points associated with each category of each risk factor are computed by: Pointsij = βi (Wij-WiREF)/B and rounded to the nearest integer. | |||||
Points were assigned to each category for each predictor (Table 2). The total risk score with a range of 0 to 33 linearly correlated with the risk estimate of CPP (r = 0.96, P < 0.0001, Table 3). The proportion of patients diagnosed as CPP for each risk score value was showed in Table 3. C-index for the risk score system was 0.85 (95% CI, 0.81–0.89; Fig. 2). Calibration plot showed an intercept of -0.02, and a slope of 1.02 (Additional file 1 [Additional Fig. 1]). Two cutoff points were selected to stratify CPP risk (low risk: < 10 points; medium risk: 10–19 points; high risk: ≥ 20 points). The proportion of CPP patient in the low-, medium-, and high-risk population was 10% (4/40), 49.8% (100/201), and 93.2% (68/73), respectively. For low-risk population (cutoff point = 10), the sensitivity was 97.8% (95% CI, 95.3% − 99.5%), specificity was 24.8% (95% CI, 18.2% − 32.6%), the LR- was 0.09 (95% CI, 0.02–0.20), and negative predictive value was 90.2% (95% CI, 79.2% − 97.7%). For high-risk population (cutoff point = 20), the specificity was 96.6% (95% CI, 92.9% − 99.2%), sensitivity was 39.6% (95% CI, 32.0% − 46.2%), the LR + was 12.0 (95% CI, 5.49–48.9), and the positive predictive value was 93.3% (95% CI, 86.5% − 98.4%; Table 4).
|
CPP risk category |
Points total |
Estimate of risk (95% CI) a |
No. with CPP/ Total No. of patients in training sample (%) |
No. with CPP/ Total No. of patients in validation sample (%) |
|---|---|---|---|---|
|
Low risk |
0 |
0.01 (0.01, 0.03) |
0/0 (-) |
0/1 (0.0) |
|
1 |
0.01 (0.01, 0.04) |
0/1 (0.0) |
0/3 (0.0) |
|
|
2 |
0.02 (0.01, 0.05) |
0/3 (0.0) |
0/0 (-) |
|
|
3 |
0.03 (0.01, 0.07) |
0/4 (0.0) |
0/6 (0.0) |
|
|
4 |
0.04 (0.02, 0.08) |
0/2 (0.0) |
0/5 (0.0) |
|
|
5 |
0.05 (0.02, 0.10) |
1/3 (33.3) |
0/1 (0.0) |
|
|
6 |
0.07 (0.04, 0.13) |
0/6 (0.0) |
0/3 (0.0) |
|
|
7 |
0.09 (0.05, 0.16) |
0/3 (0.0) |
0/3 (0.0) |
|
|
8 |
0.12 (0.08, 0.20) |
1/9 (12.5) |
0/6 (0.0) |
|
|
9 |
0.16 (0.11, 0.24) |
2/9 (22.2) |
0/11 (0.0) |
|
|
Medium risk |
10 |
0.21 (0.15, 0.29) |
4/19 (21.1) |
3/19 (15.8) |
|
11 |
0.27 (0.21, 0.35) |
5/21 (23.8) |
10/19 (52.6) |
|
|
12 |
0.34 (0.27, 0.42) |
7/28 (25.0) |
13/37 (35.1) |
|
|
13 |
0.42 (0.35, 0.49) |
10/24 (41.7) |
7/19 (36.8) |
|
|
14 |
0.50 (0.43, 0.57) |
16/29 (55.2) |
17/35 (48.6) |
|
|
15 |
0.58 (0.51, 0.65) |
14/25 (56.0) |
17/32 (53.1) |
|
|
16 |
0.66 (0.58, 0.72) |
15/19 (79.0) |
14/14 (100.0) |
|
|
17 |
0.72 (0.65, 0.79) |
9/11 (81.8) |
14/17 (82.4) |
|
|
18 |
0.78 (0.70, 0.85) |
13/15 (86.7) |
5/6 (83.3) |
|
|
19 |
0.83 (0.76, 0.89) |
7/10 (70.0) |
12/12 (100.0) |
|
|
High risk |
20 |
0.87 (0.80, 0.92) |
9/10 (90.0) |
6/6 (100.0) |
|
21 |
0.91 (0.84, 0.95) |
8/9 (88.9) |
7/8 (87.5) |
|
|
22 |
0.93 (0.87, 0.96) |
3/3 (100.0) |
5/6 (83.3) |
|
|
23 |
0.95 (0.90, 0.98) |
6/8 (75.0) |
4/5 (80.0) |
|
|
24 |
0.96(0.92, 0.98) |
10/11 (90.9) |
6/7 (85.7) |
|
|
25 |
0.97 (0.93, 0.99) |
5/5 (100.0) |
3/3 (100.0) |
|
|
26 |
0.98 (0.95, 0.99) |
8/8 (100.0) |
5/5 (100.0) |
|
|
27 |
0.99 (0.96, 0.99) |
2/2 (100.0) |
4/4 (100.0) |
|
|
28 |
0.99 (0.97, 1.00) |
4/4 (100.0) |
3/3 (100.0) |
|
|
29 |
0.99 (0.97, 1.00) |
3/3 (100.0) |
1/1 (100.0) |
|
|
30 |
0.99 (0.98, 1.00) |
2/2 (100.0) |
1/1 (100.0) |
|
|
31 |
1.00 (0.98, 1.00) |
6/6 (100.0) |
7/7 (100.0) |
|
|
32 |
1.00 (0.99, 1.00) |
0/0 (-) |
0/0 (-) |
|
|
33 |
1.00 (0.99, 1.00) |
2/2 (100.0) |
8/8 (100.0) |
|
|
|
||||
| We define the constant B for the points system (the number of regression units that will correspond to one point) as the increase in risk of CPP associated with a 0.2 (IU/L) increase in basal LH: B = 0.2*1.63 = 0.326 | ||||
|
Training Sample (n = 314) |
Validation Sample (n = 313) |
|
|---|---|---|
|
AUC (95%CI) |
0.85 (0.81, 0.89) |
0.86 (0.82, 0.90) |
|
Calibration |
a=-0.02, b = 1.02 |
a=-0.02, b = 1.06 |
|
Cutoff point = 10 |
||
|
Sensitivity (%, 95%CI) |
97.8 (95.3, 99.5) |
100.0 (-) |
|
Specificity (%, 95%CI) |
24.8 (18.2, 32.6) |
27.7 (20.2, 34.9) |
|
Positive likelihood ratio (95%CI) |
1.30 (1.20, 1.46) |
1.38 (1.25, 1.54) |
|
Negative likelihood ratio (95%CI) |
0.09 (0.02, 0.20) |
0.0 (-) |
|
Positive predictive value (%, 95%CI) |
61.2 (55.9, 67.0) |
62.6 (56.8, 68.6) |
|
Negative predictive value (%, 95%CI) |
90.2 (79.2, 97.7) |
100.0 (-) |
|
Cutoff point = 20 |
||
|
Sensitivity (%, 95%CI) |
39.6 (32.0, 46.2) |
34.8 (27.8, 42.1) |
|
Specificity (%, 95%CI) |
96.6 (92.9, 99.2) |
97.3 (94.2, 100.0) |
|
Positive likelihood ratio (95%CI) |
12.0 (5.49, 48.9) |
12.6 (5.44, 48.8) |
|
Negative likelihood ratio (95%CI) |
0.63 (0.55, 0.71) |
0.67 (0.59, 0.75) |
|
Positive predictive value (%, 95%CI) |
93.3 (86.5, 98.4) |
93.9 (86.6, 100.0) |
|
Negative predictive value (%, 95%CI) |
56.9 (50.8, 62.9) |
55.2 (48.8, 61.4) |
Validation Sample
There were 313 patients in the validation sample. C-index for the logistic regression model and risk score model was both 0.86 (95% CI, 0.82% − 0.90%) (Fig. 2). Calibration plot of the observed frequency of CPP patients against the predicted probability of CPP showed an intercept of -0.02, and a slope of 1.06, suggesting acceptable calibration (Additional file 1 [Additional Fig. 1]).The total risk score in the validation sample ranged from 0 to 33. The proportion of CPP patient in the low-, medium-, and high-risk population was 0.0% (0/39), 53.3% (112/210), and 93.8% (60/64), respectively (Table 4). The test characteristics retained in the validation sample (Table 4).