Tables
Table 1: Structural comparison of CYP81Q-related genes
DOI: https://doi.org/10.21203/rs.3.rs-885568/v1
Recent genomic studies of parasitic plants have revealed that there are numerous footprints indicative of horizontal gene transfer (HGT) to the parasites from their host plants. However, the molecular mechanisms and biological impacts of this phenomenon have remained largely unknown. Here, we made the striking observation that two parasitic dodders, Cuscuta campestris and C. australis, have functional homologues of Si_CYP81Q1, which encodes piperitol/sesamin synthase (PSS) in the phylogenetically remote plant Sesamum indicum (sesame). The apparent lack of sequence similarity between the regions flanking PSS in Sesamum and Cuscuta spp. suggests the occurrence of HGT tightly associated with the PSS gene. Upon parasitism, C. campestris induced expression of the host Si_CYP81Q1 at the parasitic interface and mature and intron-retained Si_CYP81Q1 mRNA was transferred to C. campestris, suggesting that CYP81Q1 was translocated via RNA-mediated HGT. Thus, parasitism-evoked HGT might have had an unexpected role in the metabolic evolution of plants.
Cuscuta spp. (Convolvulaceae, Solanales), commonly known as dodders, are obligate parasitic plants with a broad host range1,2. In a recent genome analysis, one species, C. australis, was estimated to be separated by about 55 MYA from the phylogenetically related morning glory (Ipomoea nil., Convolvulaceae, Solanales)3. Cuscuta plants are rootless and leafless, and thus have limited or no photosynthetic ability4. To obtain biochemical resources for survival and proliferation, Cuscuta have acquired the ability to form connections with autotrophic host plants through a specialized root-like structure called the haustorium, which enables acquisition of sugars and minerals from the host. Notably, recent studies have shown that Cuscuta plants also take in genomic DNA and messenger RNA from host plants5,6. Interestingly, the strictosidine synthase-like gene in C. australis showed a much higher sequence similarity with related genes in Brassicaceae than genes in close relatives of C. australis, suggesting HGT from Brassicaceae host plants to the parasites7. C. australis also acquired an acyltransferase gene from Fabaceae host plants8. These data suggest that the Cuscuta genome has acquired a substantial number of genes during its evolution; however, whether such HGT-derived genes in Cuscuta are functional has not been clarified, and the mechanisms by which genetic molecules are delivered from host plants to Cuscuta have not been elucidated.
Despite their lack of photosynthetic ability, Cuscuta plants are known to produce a variety of bioactive compounds. Cuscuta spp. are used in Asian traditional herbal medicine for anti-aging, anti-inflammatory, hepatoprotective, pain reliever, and aphrodisiac purposes9,10. Previous studies demonstrated that Cuscuta spp. contain a variety of specialized bioactive metabolites including flavonoids, steroids, and alkaloids10,11, which might represent adaptive metabolic evolution to specific situations and might reflect the unique physiology of these plants.
Specialized metabolites are lineage-specific and usually restricted to a phylogenetically close group, known as a chemotaxonomic group, due to the common biosynthetic origin of an ancestor e.g., isoflavonoid in Fabaceae. However, some specialized metabolites are sporadically found in phylogenetically unrelated plants, forming metabolic ‘patchiness’ in chemotaxonomy. The sporadic occurrence of the specialized metabolite can be explained by convergent evolution (CEV) based on the independent occurrence of biosynthetic genes, which produce common metabolites in several plants. For example, a specialized metabolite derived from xanthine, caffeine, can be found not only in Camellia sinensis (tea) and Coffea arabica (coffee), which are both in the Asterid group, but also in Theobroma cacao (cacao) and Citrus sinensis (orange), which are in a distinct clade, the Rosids. The discontinuous presence of caffeine has been explained as CEV of caffeine synthase genes12. More recently, CEV of a biosynthetic gene cluster for momilactone biosynthesis was uncovered in different plant lineages13, indicating that CEV is the genetic basis for sporadically distributed metabolites in plants.
Sesamin is another example of a sporadically occurring specialized metabolite. Lignans are a large class of phenylpropanoid-dimeric metabolites found in plants. Sesamin is the major lignan in seeds of Sesamum spp. (Pedaliaceae, Lamiales) and is also found in phylogenetic relatives of Sesamum, e.g. Pedaliaceae and Paulowniaceae14. In sesame seeds, a cytochrome P450 monooxygenase of S. indicum, Si_CYP81Q1, also known as piperitol/sesamin synthase (PSS), forms two methylenedioxy bridges (MDB) on the two aromatic rings in the sequential conversion of pinoresinol to piperitol and then to sesamin15. The strikingly homologous CYP81Q genes are functionally conserved in phylogenetic relatives of S. indicum, which produce sesamin and its related metabolites15,16,17. This is an example of a typical metabolic radiation within a restricted taxonomic group sharing a common biosynthetic gene.
In addition to being found in Lamiales, sesamin is also discontinuously observed in phylogenetically unrelated plants such as Piper spp. (black pepper), Magnolia spp. (a basal angiosperm), and Ginkgo (Gymnosperm)14,18,19. Given the lineage specificity of specialized metabolites, it is remarkable that Cuscuta spp. commonly contain sesamin and related metabolites such as cuscutoside (Supplementary Fig. 1), since thus far, sesamin has not been reported in Convolvulaceae other than Cuscuta9,14,20,21,22. Notably, sesamin is detected in Cuscuta palaestina, which is a parasite of host plants that do not accumulate sesamin, suggesting that C. palaestina de novo produce sesamin in planta rather than absorbing it from host plants22. Thus, sesamin serves as an excellent example of the sporadic occurrence of a specialized metabolite. Nevertheless, how Cuscuta acquired the ability to produce sesamin has not yet been elucidated (Fig. 1a).
The PSS genes in Lamiales are the only genes known to encode proteins with sesamin synthase activity. There are at least three possible explanations for the sporadic occurrence of sesamin: 1) functional differentiation or gene loss (FD/GL), 2) CEV of a sesamin synthase, and 3) HGT of a sesamin synthase gene. A previous report on FD of sesamin synthase showed that S. alatum CYP81Q3 produces pluviatilol instead of sesamin, resulting in the exceptional absence of sesamin in S. alatum among the Sesamum spp.17. FD/GL is likely restricted to a relatively small taxonomic group due to its dependence on metabolic radiation coupled with a common biosynthetic gene. By contrast, CEV occurs in distant, unrelated phylogenies, free from metabolic radiation. As a result, CEV genes tend not to be similar due to their distinct genetic origins. HGT provides an alternative explanation for the presence of sporadic metabolites and likely occurs in parasitic plants such as Cuscuta, Striga, and Orobanche spp. via the unusual physical interaction these plants have with their hosts. In this case, HGT genes are expected to be structurally similar to genes originally encoded in host plants. Identification of sesamin synthase genes from non-Lamiales plants would not only clarify the metabolic origins of sesamin but also shine new light on the nature of metabolic evolution of specialized metabolites.
In this study, we attempt to unravel the origin in Cuscuta plants of sesamin and structurally related metabolites with MDB by integrating comparative genomics, parasitism testing, and biochemical approaches. The results obtained using these different approaches support the unexpected finding that parasitism-mediated functional HGT is a possible driving force for sporadic metabolic evolution.
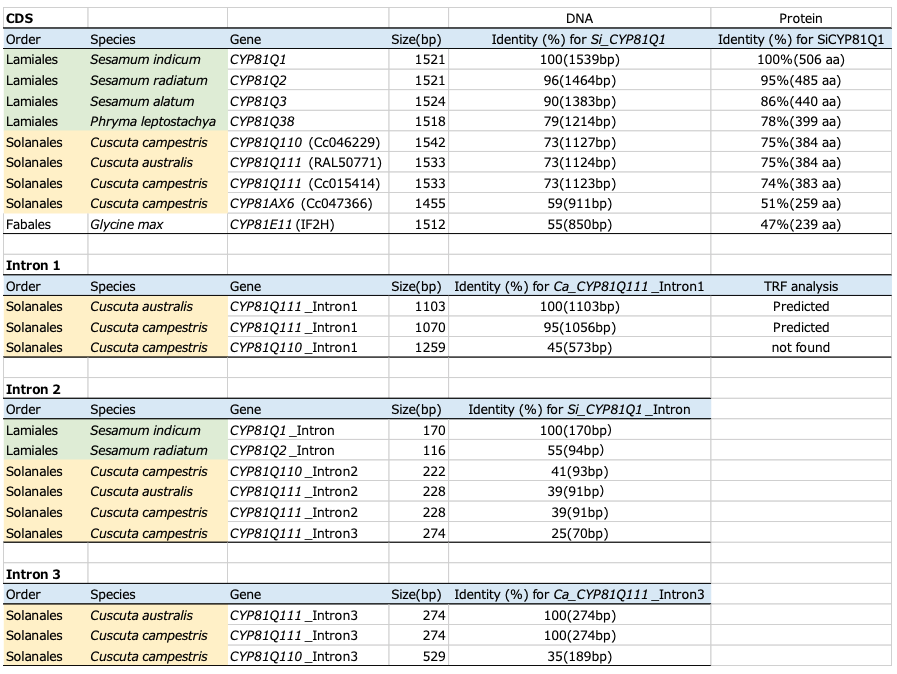
We analyzed lignans in C. campestris seeds and detected sesamin and related lignans (Supplementary Fig. 1). To elucidate the molecular basis of sesamin biosynthesis in Cuscuta plants, we investigated genome and transcriptome data of C. campestris by Basic Local Alignment Search Tool (BLAST) search using Si_CYP81Q1 (AB194714) as a query23. We found two CYP81Q1 homologs, Cc_CYP81Q110 (Cc046292) in scaffold18 and Cc_CYP81Q111 (Cc015414) in scaffold84. Both of these genes are predicted to have three introns, their putative amino acid sequences are 95% identical, and they share ca. 75% identity with Si_CYP81Q1 (Fig. 1b, Table 1, Supplementary Table 1). We identified Cc_CYP81AX6 (Cc047366) as a third homolog of CYP81Q1 in the C. campestris genome; however, Cc_CYP81AX6 has relatively low amino acid identity to Si_CYP81Q1 (51%) and formed a distinct phylogenetic cluster together with isoflavone hydroxylases from Fabaceae such as Ge_CYP81E124,25 and CYP81E-related genes from I. nil. This cluster is distinct from that harboring Cc_CYP81Q110 and Cc_CYP81Q111. By contrast, no genes with striking similarity to Si_CYP81Q1 were observed in the I. nil genome or transcriptome26.
We also found a single P450 gene, Ca_CYP81Q111 (C065N002E0.1), that has high structural similarity to Si_CYP81Q1 (75% amino acid identity) in another (+)-sesamin producing dodder, Cuscuta australis, which is phylogenetically related to C. campestris and whose genome has been sequenced (Fig. 1b, Table 1, Supplementary Table 1)3,14. The size of the C. australis genome (c.a. 264 Mbp) is approximately half of that of C. campestris (c.a. 556 Mbp). This is consistent with the idea that the C. campestris genome doubled in size due to either a recent whole genome duplication (WGD), estimated to have occurred in the C. campestris genome around 1.5 MYA after its divergence from C. australis or via a hybridization event between phylogenetic relatives2,3,21,23. Based on this, we concluded that Ca_CYP81Q111 is singlet in the genome, whereas Cc_CYP81Q110 and Cc_CYP81Q111 are twin homeologs.
We next used a bioinformatics approach to try to construct the DNA sequences of Cuscuta CYP81Q-related genes using public NGS data of the following Cuscuta plants: C. californica, C. gronovii, and C. americana from subgenus Grammica; C. europaea and C. epithymum from subgenus Cuscuta; and C. reflexa, C. japonica, and C. monogyna from subgenus Monogynella, which is the most primitive group of Cuscuta and is similar to nonparasitic relatives1. Partial sequences including exon 2 of Cuscuta CYP81Q genes were found by BLAST search in Grammica and Cuscuta, but not in the three species of Monogynella (Supplementary Fig. 2a). The absence of CYP81Q-related genes was experimentally examined by PCR using primers designed for exon 2. No amplification was observed from genomic DNA of C. japonica belonging to Monogynella as a template while a specific band was amplified from that of C. campestris belonging to Grammica (Supplementary Fig. 2b). Additionally, sesamin was not detected in seeds of C. japonica (Supplementary Fig. 1), consistent with the idea that there is no CYP81Q-related gene in this species. The three exon 2 sequences from subgenus Grammica clustered with those of C. australis and C. campestris, whereas the two sequences of subgenus Cuscuta formed a phylogenetic clade distinct from that of Grammica, roughly in line with the phylogeny of Cuscuta spp.
These results support the notions that 1) the CYP81Q-related gene is widely conserved among subgenera Grammica and Cuscuta, 2) the origin of this gene likely ascends to a monophyletic ancestor located between subgenera Monogynella and Cuscuta, and 3) the gene has undergone nucleotide diversification from the common ancestral CYP81Q orthologue along with speciation of the subgenera Cuscuta and Grammica.
In contrast to Cuscuta spp., we did not find any homologs of Si_CYP81Q1 in the public genomes of Ginkgo biloba, Magnolia ashei, or Piper nigrum in BLAST search, despite the fact that their phylogenetic relatives produce sesamin. This result is consistent with previous notions regarding plant P450 phylogeny, i.e. that proteins in the CYP81 family have not been conserved in basal angiosperms and gymnosperms27. Considering that these plants are phylogenetically very distant from Lamiales, their sesamin synthase genes, which are as yet unidentified, are thought to be independent in origin from Si_CYP81Q1 and probably occurred through CEV (Fig. 1a).
It should be noted that Si_CYP81Q1 has a unique Ala (Ala308) residue that is crucial for catalysis of MDB formation in its distal I-helix, where among P450s, a conserved Thr residue (distal-Thr) is commonly located (Supplementary Fig. 3)16,28. Each of the three Cuscuta P450s, Cc_CYP81Q110, Cc_CYP81Q111, and Ca_CYP81Q111, also has an Ala residue (Ala313, Ala310, and Ala310, respectively), at the site corresponding to the distal-Thr residue (Supplementary Fig. 3). These data indicate that Cc_CYP81Q110, Cc_CYP81Q111, and Ca_CYP81Q111 satisfy the structural requirements to be considered putative MDB-forming enzymes and most likely catalyze the production of sesamin.
Structural conservation of CYP81Q1-related genes within Cuscuta spp. suggests that they have common biochemical functions in planta. To evaluate biochemical properties of the Cuscuta CYP81Q genes, recombinant proteins were co-expressed with a C. campestris cytochrome P450 reductase, Cc_CPR1 (Cc043955), in a yeast system29. When fed with (+)-pinoresinol, Cc_CYP81Q110, Cc_CYP81Q111, or Ca_CYP81Q111 formed two MDBs on the two aromatic rings of the (+)-pinoresinol and produced (+)-sesamin via (+)-piperitol (Fig. 2). The stereochemistry of the (+)-sesamin produced by the three Cuscuta CYP81Q genes was identical to that generated by Si_CYP81Q1 using (+)-pinoresinol as a substrate. Moreover, they showed trace levels of MDB-forming activity for (+)-epipinoresinol and (-)-pinoresinol. The results of LC-MS analysis revealed that the product formed from (+)-epipinoresinol is likely to be a piperitol isomer, (+)-pluviatilol, which has a single MDB, and that the product from (-)-pinoresinol is (-)-sesamin, which has two MDBs. In contrast, the third CYP81 family protein found in C. campestris, Cc_CYP81AX6, did not show MDB-forming activity for any of the pinoresinol isomers tested in this study (Supplementary Fig. 4). Collectively, the data show that the three Cuscuta CYP81Q genes encode functional PSS and provide a molecular basis for the presence of sesamin in Cuscuta spp.
Open-source RNA-seq analyses showed that the two Cc_CYP81Q genes are coordinately expressed in seedlings and flower bud clusters (Fig. 3a)30, whereas Ca_CYP81Q111 was expressed in buds, ovaries and seeds but not in germinating seedlings (Fig. 3b)3. Moreover, we detected the presence of (+)-sesamin in C. campestris in both fruits containing seeds and flower bud clusters grown on Nicotiana tabacum as a non-sesamin producing parasitic host (Supplementary Fig. 1). This is consistent with the expression profiles of the two C. campestris PSS genes. By contrast, the third homolog in C. campestris, Cc_CYP81AX6, showed negligible expression in these organs. Collectively, these results show that the sesamin found in Cuscuta plants is de novo synthesized in reproductive organs and seeds, rather than produced by the host plant and transported to Cuscuta plants.
The high structural similarity and functional conservation between sesame and Cuscuta CYP81Q genes prompted us to estimate the time of divergence these P450 genes using the RelTime method31. The point of divergence of sesame and Cuscuta CYP81Q1 genes was calculated to be 47.8 MYA using the proportional mode (constant rate) method calibrated to the time of divergence between sesame and olive (Olea europaea), both of which are Lamiales, which was calculated to be 81 MYA (Supplementary Table 2, Supplementary Fig. 5). By contrast, the point of divergence of sesame and Cuscuta plants was estimated to be 84 MYA16. This temporal discrepancy in the estimation of divergent time among the Cuscuta CYP81Q genes as compared with lineage speciation suggests a recent origin for the PSS genes, i.e. after speciation of Cuscuta.
We next amplified the genomic regions containing CYP81Q genes from S. indicum and C. campestris using genomic DNA as a template and specific PCR primer sets. Then, we empirically confirmed the sequence of the PCR-amplified fragments by sequencing and found that both Cc_CYP81Q110 and Cc_CYP81Q111 consist of four exons separated by introns (three introns total), as predicted based on whole-genome sequence data. In contrast, Si_CYP81Q1 has two exons with a single intron15,33. The first and the third introns in the two Cc_CYP81Q genes were highly conserved (Fig. 1c). Since the overall genomic structure of these two Cc_CYP81Q genes was also conserved in another CYP81Q-related gene, Ca_CYP81Q111 found in C. australis genome3, generation of the first and the third introns of CYP81Q1 genes in Cuscuta plants might have occurred prior to speciation of C. campestris and C. australis. Importantly, the position of the second intron of the three Cuscuta CYP81Q genes was identical to that of the intron in Si_CYP81Q1. By contrast, Cc_CYP81AX6 had only a single intron and thus, showed a different genomic structure (a single intron and two exons). These common genomic signatures among CYP81Q genes observed between distantly-related plants, i.e. Cuscuta and Sesamum, implies a shared origin for these cytochrome P450 genes.
To further evaluate genomic features of CYP81Q genes, we compared nucleotide sequence similarity of introns from sesame and dodder by CLUSTALW alignment (Table 1). The intron of Si_CYP81Q1 (170 bp) and intron 2 of the three Cuscuta CYP81Q genes (222–228 bp) shared approximately 40% nucleotide sequence identity, whereas the intron of Si_CYP81Q1 and intron 3 of Cc_CYP81Q111, which is comparable in length to intron 2 (275 bp), shared only 25% identity, a level comparable to random. These results highlight structural similarity between the intron of Si_CYP81Q1 and intron 2 of the three Cuscuta CYP81Q genes. Structural comparison among intron 1 or intron 3 of Cuscuta CYP81Q genes revealed structural divergence of Cc_CYP81Q110 from the highly homologous Cc_CYP81Q111 and Ca_CYP81Q111, each of which was predicted to have a repetitive sequence in intron I (Table 1)34. Moreover, we detected DNA transposons (Stowaway and hAT) in introns of Cuscuta CYP81Q genes (Fig. 1) 35,36. Cc_Sto1 and Cc_Sto2 were located in introns 1 and 3 of Cc_CYP81Q110, respectively, whereas Cc_hAT1 was located in intron 1 of both Cc_CYP81Q111 and Ca_CYP81Q111 (Supplementary Fig. 6). Intriguingly, the characteristic footprint sequence of Stowaway was detected in introns 1 and 3 of both Cc_CYP81Q111 and Ca_CYP81Q111 at the locations corresponding to where Cc_Sto1 and Cc_Sto2 are located in Cc_CYP81Q110.
We next compared gene synteny in the regions surrounding the CYP81Q genes in S. indicum, C. campestris, and C. australis (Fig. 4). In the S. indicum genome, Si_CYP81Q1 (SIN_1025734) is located on chromosome 9, between ATPase (SIN_1025733) and an uncharacterized protein (SIN_1025735) next to the FLOWERING LOCUS C expresser (FLX)-like gene (SIN_1025736). By contrast, Cc_CYP81Q110 and Cc_CYP81Q111 were both located in reverse orientation as compared to their neighboring genes, in the vicinity of an E3-class ubiquitin ligase (E3UbL). Specifically, Cc_CYP81Q110 is between DUF4228 (Cc046290) and a hypothetical protein (Cc046293), and Cc_CYP81Q111 is between DUF4228 (Cc015411: gene number 1, Fig. 4) and another hypothetical protein (Cc015416: gene number 9, Fig. 4, Supplementary Table 1). Moreover, these genomic features were also observed nearby the RAL50776 gene in the C. australis genome. The observation that the genomic features in introns and the region adjacent to Cc_CYP81Q111 were distinct from those of Cc_CYP81Q110 and Ca_CYP81Q111 (Figs. 1 and 4) suggests that the twin homeologs in C. campestris were brought by hybridization (allopolyploidization) between two Cuscuta plants, both of which had the ability to produce sesamin, rather than occurring through WGD of an ancient C. campestris genome (autopolyploidization).
Although multiple E3UbL genes were observed between DUF4228 (LOC109177929: gene number 1, Fig. 4) and a hypothetical protein (LOC109177844: gene number 9, Fig. 4) in chromosome 5 of the I. nil genome, there were no cytochrome P450 genes in these regions of the three Ipomoea genomes corresponding to the Cuscuta PSS genes. This is notable since the arrangement of genes between DUF4228 and LOC109177844 in the I. nil genome is structurally analogous to those between Cc046290 and Cc015411, and between Cc046293 and Cc015416, in the C. campestris genome.
Collectively, comparative genomics approaches based on gene synteny across species highlight the unique presence of CYP81Q genes in Cuscuta plants, supporting the idea that CYP81Q genes appeared in the Cuscuta genome by HGT. We cannot exclude the alternative possibility that an ancestor of I. nil had a CYP81Q1 ortholog but lost it during speciation, although aside from Cuscuta, no other Convolvulaceae plants are known to accumulate sesamin14,37.
The observance of PSS genes with conserved genomic features and molecular functions in Sesamum and the distantly-related parasitic Cuscuta plants suggests that HGT of PSS occurred through parasitism between their ancestors. To help explore the feasibility of this model of HGT, we examined whether C. campestris is able to parasitize S. indicum by co-cultivating C. campestris with S. indicum (cv. Masekin) under laboratory conditions. The results showed that C. campestris successfully formed haustoria on the stem of S. indicum 2–3 weeks after germination of the parasites (Fig. 5a). Cross sections at the haustorium revealed that the vascular tissues of S. indicum were directly connected with haustorial vascular elements of the parasite (Fig. 5b). At 8–9 weeks after germination, C. campestris formed a flower and developed an ovary that contained fertile seeds, thereby completing its lifecycle as an obligate parasite (Fig. 5c, d). The ability to parasitize the modern sesame cultivar supports the model that parasitism by a Cuscuta of a Sesamum, or a phylogenetically related plant with PSS, facilitated HGT.
The presence of intron 2 in all of the Cuscuta PSS genes (Fig. 1c) indicates that the putative HGT event likely occurred via transfer of a genomic fragment containing an ancestral CYP81Q gene with the intron, rather than via retroposition of an intron-less mRNA. To test the possibility of transfer of a genomic fragment, we analyzed sequence similarity between the genomic regions flanking CYP81Q genes of Cuscuta plants and S. indicum, and found that they shared extremely low similarity (Supplementary Fig. 7). This result does not further support the idea of transfer of a long genomic fragment to Cuscuta and is different from HGT events reported in Orobanchaceae, in which transferred genomic fragments are typically tens of kbp in length38. Nevertheless, we cannot exclude the possibility that in Cuscuta, a long genomic fragment was inserted first and then the sequence of regulatory regions changed, probably due to lower genetic constraints in those regions than in coding regions.
Acquisition of CYP81Q genes in the genome of the ancestor of Cuscuta might also have been facilitated by RNA-mediated HGT, as has been predicted in a root parasitic plant, Striga hermonthica39. In support of this idea, we note that (1) RNA molecules harboring introns (known as intron retention; IR) have been recognized to exert biological functions in plants and mammals40,41, and (2) RNA-based HGT likely occurs more readily when mRNA abundance increases since mRNA abundance might be a determinant of long-distance mobility42, whereas cellular genomic DNA content is relatively static. To test the hypothesis that RNA-based HGT accounts for the presence of CYP81Q, we surveyed IR-RNA of CYP81Q1 using S. indicum RNA-seq data29,43,44 and found this form of the RNA among minor transcripts (< 3% of total transcripts) in seeds of cv. Masekin (Supplementary Fig. 8). We also found evidence of the three components of a plant polyadenylation signal, including a near upstream element (NUE; AAUAA) and a 1 nt variant of the NUE (AAUACA); a 1 nt variant of the far upstream element (FUE; TTGTAA); and UGUA-containing hexamers following a short poly(A) stretch45, in the 3’ sequences downstream of the stop codons of Cuscuta CYP81Q genes46 (Supplementary Fig. 9). Moreover, both Cuscuta CYP81Q111 transcripts were found to harbor a short poly(A) stretch. These sequences seemed to be structurally incomplete but might be fading genomic signatures of an ancient RNA-mediated HGT event.
Next, we used qPCR and RNA-seq to look at gene expression of Si_CYP81Q1 in the host stem, where Cuscuta specifically forms haustoria. Si_CYP81Q1 was expressed at minute levels in the non-parasitized host stem. Surprisingly, Si_CYP81Q1 was significantly induced by parasitization of C. campestris, but not by mechanical wounding (Fig. 5e-g), suggesting that induction of Si_CYP81Q1 expression in S. indicum is not a response to wounding caused by the penetration of haustoria, but a specific host defense response against the parasite. Both spliced and IR-RNA forms of Si_CYP81Q1 transcripts were induced, and the ratio of IR-RNA (intron/exon) did not change after parasitism (Fig. 5e-g). We could occasionally detect both spliced and IR-RNA forms of Si_CYP81Q1 in the stem of C. campestris that had established parasitic connections to S. indicum (Fig. 5h). Sequences of the shorter and longer amplified fragments were confirmed to be the spliced and IR forms of host Si_CYP81Q1 transcripts, respectively, indicating translocation of induced RNAs of Si_CYP81Q1 to parasite stem from the parasitic interface of the host stem through the haustorium. Long-distance movement of various types of RNA between plants has been reported in interspecific grafts47 and parasite-host plant complexes5, 48, suggesting the involvement of vascular transport of RNAs. The possibility of transport of RNAs by nonenveloped RNA viruses, which encapsidate host RNAs, has also been suggested49. Our results support an evolutionary scenario in which functional HGT of PSS into the parasite genome was mediated by parasitism-evoked locally abundant RNAs. This idea is consistent with the fact that only one Cuscuta CYP81Q gene encodes a functional lignan catalytic unit in the conserved genomic synteny among Convolvulaceae plants (Fig. 4).
The presence of sesamin and its derivative lignans in the seeds of distantly related plants suggests that there are common but unknown roles for sesamin and derivative lignans in these seeds. One such role might be to prevent lipid peroxidation by exerting their (pro)antioxidative activities33, thereby preserving storage resources in oil bodies for biogenesis in the next generation. This trait conferred by seed lignans seems also to be beneficial for survival of Cuscuta seedlings, which entirely rely on the nutritive reserves stored in the endosperm until they start sucking nutrition from host plants via haustoria50. Furthermore, a sterol-binding dehydrogenase found in oil bodies, known as steroleosin (Sol), is widespread in seed plants including gymnosperms, and is involved in germination and development by regulating phytohormone sensitivity51,52. A recent report using nano-beads with affinity to sesamin identified Sol-B as a sesamin binding protein and indicated that the interaction between Sol-B and sesamin is physiologically relevant in developing seedlings53. Using S. indicum Sol-B in a BLAST search to query Cuscuta genomes, we found Sol-B-like genes (Cc036318.t1, Cc036490.t2, and C002N0117E0.1) in Cuscuta and an partial Sol-B gene amplified by PCR from genomic DNA of C. japonica that show striking structural similarity to those of Ipomoea but not those of Sesamum, roughly in line with the evolutionary plant lineages (Supplementary Fig. 10a). Thus, Sol-B genes seems to have gradually diverged along with plant speciation, again highlighting the unusual similarity among CYP81Q genes in Sesamum and Cuscuta (Fig. 1b). We further confirmed co-expression of Cc_Sol-B and Cc_CYP81Q in C. campestris seeds such as S. indicum (Supplementary Fig. 10b and c)53, suggesting common roles via interaction between sesamin and Sol-B protein in their seeds. Whether HGT-mediated metabolic traits acquired from host plants has contributed to the unique physiology and ecology of Cuscuta specifically remains an open question that can be addressed in subsequent studies.
Recent reports have described several genomic signatures of nuclear HGT from hosts to parasites but the functions of genes transferred to parasitic plants have remained largely elusive7,23,38,39,54,55. In this work, we identified that PSS genes in C. campestris and C. australis are biochemically functional and we provide a possible explanation based on genomic analysis as to how PSS genes were integrated into the genomes of Cuscuta spp. Our findings support the idea that a common ancestor of the Cuscuta and Grammica subgenera in the Cuscuta genus gained a functional genomic PSS gene from an ancestor of S. indicum through parasitism-mediated HGT, rather than, as generally assumed, by CEV, and thereby acquired (+)-sesamin biosynthetic ability (Fig. 6, Supplementary Fig. 11). Furthermore, we suggested a possible mode of parasitism-mediated HGT from the host plant to the parasitic plant via locally induced gene expression in the parasitic interface by uncovering genomic signatures of parasite Cuscuta CYP81Q genes, as well as parasitism-mediated induction and transfer of host Si_CYP81Q1 transcripts into the parasite.
Our findings herein underscore that in addition to CEV, parasitism-mediated HGT provides another possible driving force for the sporadic occurrence of specialized metabolites in the plant kingdom, as has been observed for microorganisms. Furthermore, we note that Cuscuta plants are obligate parasites and can parasitize a wide variety of host plants (Supplementary Fig. 12)6,23,56, and suggest that there might be more opportunities for HGT to occur in such parasitic plant species than in non-parasitic plants. The biological significance of the presence of (+)-sesamin-derived lignans in Cuscuta and the molecular mechanism of HGT from host to parasite remain unclear. However, evidence of parasitism-mediated HGT of PSS in Cuscuta plants provides a new perspective on metabolic evolution in plants beyond typical phylogenetic constraints and reproductive isolation, and establishes these parasites as new tools for investigating the biological activities of specialized metabolites. The unique ecological nature of Cuscuta plants as mediators of genetic and chemical information, given their promiscuous host range and ability to disperse seeds at long distance, including across oceans1,2, suggest that they might play important though as yet to be elucidated roles in plant evolution and adaptation47.
Plant materials
Cuscuta campestris seeds were germinated and seedlings were parasitized onto host plants as described previously57. Nicotiana tabacum plants were grown and parasitized by C. campestris as described previously51. The C. campestris-N. tabacum parasitic complexes were grown at 25°C under a 16/8 h light/dark cycle, and flowers and fruits of the C. campestris were harvested. The harvested tissues were frozen in liquid nitrogen and stored at -80°C. Seeds of Sesamum indicum cv. ‘Masekin’ were germinated and grown in soil (Sukoyakabaido, Yanmar, Osaka, Japan) mixed with the same volume of vermiculite (GS30L, Nittai Co., Ltd., Aichi, Japan) under natural sunlight illumination from May to August in 2019. Mature stems of 8- to 9-week-old S. indicum were parasitized by C. campestris.
Chemicals
(+)-Sesamin (Chromadex), (+)-sesaminol (Nagara Science), (+)-pinoresinol (Sigma-Aldrich), and (+)-piperitol, synthesized previously16, were prepared as the reference samples.
LC-MS analysis
Lignans in extracts of flowers and seeds of Cuscuta plants (C. campestris and C. japonica) were analyzed as follows. A 10 mg sample of lyophilized flowers or seeds was homogenized to a fine powder using a TissueLyser II (Qiagen). Next, 1 ml of 70% acetonitrile aqueous solution was added to the homogenized samples and the samples were extracted using an ultrasonic cleaner at room temperature for 2 min. The filtered extracts were analyzed using an ion-trap time-of-flight mass spectrometer (LCMS-IT-TOF, Shimadzu) equipped with a photodiode array detector (Shimadzu). Each component was separated using a YMC Triart C18 column (TA12S03-1503WT, 150 mm × 3 mm I.D., 3 µm P.S.) with mobile phases A, 0.1% HCO2H-H2O; and B, 0.1% HCO2H-MeOH, in a linear gradient elution with 30-50-90-90-30-30% B (0-10-25-32-32.01-40 min) at a flow rate of 0.3 ml/min.
PCR
Genomic DNA from C. campestris (Cc), C. japonica (Cj) and S. indicum (Si) was prepared from stems using the DNeasy Plant Mini kit (Qiagen) according to the manufacturer’s protocol. Total RNA was prepared using the RNeasy Plant Mini kit (Qiagen) from the following tissue following lysis using a TissueLyser II (Qiagen): Cc seeds, Cc seedlings (at 7 days after germination), Si stem-Cc haustorium junction (parasitic interface tissues), Cc stems, Si stems with and without mechanical wounding with a blade, and Si leaves. The RNA samples were treated with DNase Set (Qiagen) to eliminate contaminating genomic DNA. After DNase I treatment, cDNA was synthesized using an oligo dT primer and the PrimeScript RT reagent kit (TAKARA BIO). Reactions were performed according to the manufactures’ instructions. PCR products were amplified using specific primer sets (listed in Supplemental Data 4) as described previously17,58,59,60. Briefly, genomic PCR, qPCR and RT-PCR was conducted using PrimeSTAR Max DNA Polymerase (TAKARA BIO), GoTaq qPCR Master Mix (Promega) and PrimeSTAR GXL DNA Polymerase (TAKARA BIO), respectively.
RNA extraction for RT-PCR and RNA-seq
Total RNA for RT-PCR was prepared from S. indicum (Si) CYP81Q1 in C. campestris (Cc) stems and total RNA for RNA-seq was prepared from Cc seedlings, Si-Cc haustorium junctions (parasitic interface tissues), Si-Cc Si leaves, Cc stems growing on the Si stem, Si stems with and without mechanical wounding with a blade, and Si leaves. Cc seedlings were harvested 7 days after germination, which was grown under 16h light/ 8h dark cycle for 5 days, blue-light illumination for 1h, under dark condition for 23h, and a 16/8 h light/dark cycle 1 day. Si plants were used 4-6 weeks and 12-16 cm height as the parasitized plants and as the plant samples with and without mechanical wounding. After parasitization or mechanical wounding, plant samples were under blue-light illumination for 1h, under dark conditions for 23h, and a 16/8 h light/dark cycle for 5 days. The samples were harvested 6 days later after parasitization or mechanical wounding. Parasitic interface tissues were harvested from 1.5 cm length include the parasitic interface. Si stems with mechanical wounding were harvested from 1.5 cm length including the cutting point. Si stems without mechanical wounding were harvested from a 1.5 cm length of epicotyl. To collect Si leaves, the topmost portions of leaves that had grown to at least 3 cm were chosen. Cc stems were harvested at 10.5 -13.5 cm length from 1.5 cm near the parasitic interface to the Cc stem tip to avoid cross-contamination with the host. Harvested tissues were washed twice with 70% EtOH for 2 min and rinsed with nuclease-free water for 2 min to clean the surface of the tissues. Total RNA was extracted using the RNeasy Plant Mini kit (Qiagen) after lysis with a TissueLyser II (Qiagen) and then treated using the TURBO DNA-free Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Each RNA sample was derived from a single organism.
Genome and transcriptome
We used open source Cuscuta transcriptome and genome data sets. The RNA-seq data for C. campestris were obtained from the DNA Data Bank of Japan Sequenced Read Archive accession number DRA009453 (https://trace.ddbj.nig.ac.jp/dra/index_e.html/DRA009453)30. The assembled genome sequence and annotations for C. campestris were obtained from the plaBi database (http://plabipd.de/portal/cuscuta-campestris) and for C. australis from the National Center for Biotechnology Information (NCBI) Sequence Read Archive (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA394036). Cc_CPR1, Cc_CYP81Q110, Cc_CYP81Q111, Cc_CYP81AX6, and Ca_CYP81Q111 correspond to Cc043955, Cc046292, Cc015414, Cc047366, and RAL50776 (responsible for C65N0022E0.1), respectively. The DNA-seq data for C. americana was obtained from SRA experiment ERR3569498 of BioProject PRJEB34450 and for C. californica, from SRA experiment ERR3569499. Synteny analysis was performed using BLASTP to search for best hit protein sequences in the databases indicated in Supplementary Table 3. All translation products of the genes listed in Supplementary Table 3 were used as queries to search all of the databases.
Molecular phylogenetic analysis
The nucleotide sequences of Cuscuta CYP81-related genes used in this study are listed in Supplementary Table 4 and Supplementary Data 1. The phylogenetic trees shown in Fig. 1, Supplementary Fig. 2, and Supplementary Fig. 9 were constructed using a maximum likelihood (ML) method in the Seaview software (phyML ln(L)=-23653.0, 1868 sites, GTR 4 rate classes)61. The timetree shown in Supplementary Fig. 5 was inferred by applying the RelTime method31, 62 to a phylogenetic tree whose branch lengths were calculated using the ML method and the Tamura-Nei substitution model. Evolutionary analyses were conducted in MEGA X software63.
Genome comparisons
The 10 kb genomic sequence containing the CYP81Q-related gene in the S. indicum genome was compared to CYP81Q-related genes in Cuscuta genomes using nucmer in the Mummer -v3.1.0 package64. Structural similarities were visualized with dotplots using the DNAnexus Dot browser (available at https://dnanexus.github.io/dot/) and Unipro UGENE software (http://ugene.unipro.ru/) (Supplementary Fig. 6).
Molecular cloning
Cc_CYP81Q110 (Cc15414), Cc_CYP81Q111 (Cc046292), and Cc_CYP81AX6 (Cc047366) were amplified by reverse transcription-polymerase chain reaction (RT-PCR) from cDNA prepared from a mixture of flower buds and ovary tissue from C. campestris with the specific primers described in Supplementary Table 4. Ca_CYP81Q111 was artificially synthetized without codon-optimization (Eurofins Genomics), based on the sequence of RAL50776. All of the P450 genes were cloned into yeast expression vectors and heterologously co-expressed in yeast with a C. campestris cytochrome P450 reductase, Cc_CPR1 (Cc043955), as described previously29.
Biochemical analysis
Biochemical analysis of Cuscuta Cc_CYP81Q110, Cc_CYP81Q111, Cc_CYP81AX6, and Ca_CYP81Q111 was performed basically as described previously29. Briefly, yeast cells expressing Cuscuta CYP genes were pre-cultured overnight at 30°C with rotary shaking at 120 rpm in 3 ml synthetic defined liquid medium containing a set of amino acids appropriate for the designated expression vectors. Next, 50 µl of stationary phase culture was transferred to 1 ml of fresh medium in 24-well plates supplemented with 100 µM of lignans as substrates. The cultures were further incubated for 24 h at 30°C with rotary shaking at 120 rpm. For extraction, the cells were harvested with the medium and disrupted by sonication. The homogenate (50 µl) was mixed with 50 µl acetonitrile and centrifuged at 21,000×g for 10 min. The supernatant was collected, filtered through a Millex-LH syringe filter (Merck Millipore) and subjected to the following HPLC analysis. Briefly, the filtered assay products were separated using a Cortecs UPLC C18+ column (part# 186007401, 2.7 µm, 3 mm × 75 mm, Waters) with mobile phases (A; 0.1% trifluoroacetic acid-H2O, and B; 0.1% trifluoroacetic acid-acetonitrile) in a linear gradient with 30-80-80-30-30% B (0-1.4-1.8-2.0-2.5 min) at a flow rate of 1.25 ml/min, and lignans were detected using a photodiode array detector at 280 nm.
Preparation and imaging of agarose-embedded sections
Parasitic interface tissues were fixed with 4% (w/v) paraformaldehyde in phosphate buffer solution (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), embedded in 8% (w/v) agarose, and cut using a MicroSlicerTM ZERO 1N (DOSAKA, Kyoto, Japan) into 200-μm sections. Histochemical staining of sections was performed using a 0.5% (w/v) solution of Toluidine Blue O (1B-481, Waldeck GmbH & Co., Munster, Germany) in distilled water. Stained slices were observed using a BX53 Biological Microscope (Olympus, Tokyo, Japan).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements:
We thank D. Nelson (Univ. of Tennessee), T. Umezawa (Kyoto Univ.), Y. Tobimatsu (Kyoto Univ.), S. Yoshida (NAIST), K. Shirasu (RIKEN), H. Satake (SUNBOR), K. Shimamoto (SUNBOR), N. Okitsu (SIC), M. Takagi (SST), Y. Kawai (NCGM), M. Yamamoto (Toyama Univ.), Y. Ogata (Osaka Pref. Univ.), K. Nishitani (Kanagawa Univ.), and all the participants in the Society of Post Youth Agronomists (SPYA 2019, at Ogoto, Shiga pref. Japan) and the Frontier Research Society of Plant Specialized Metabolism (FPSM 2019, at Matsumoto, Nagano pref. Japan) for helpful comments on and support for this work. Computations were performed in part on the NIG supercomputer at ROIS National Institute of Genetics.
Funding
This work was partly supported by Grants-in-Aid for Scientific Research (18H03950 and 19H00944, JSPS to K.A.) and a Grant-in-Aid for JSPS Fellows (19J14848, JSPS to KS).
Table 1: Structural comparison of CYP81Q-related genes
