Results
Characteristics of the included studies
A total of 17 studies including 1123 subjects were ultimately included for analysis (Fig. 1). Of these, seven studies (329 subjects) involved patients with a history of breast cancer. There were three types of treatment involved, including traditional acupuncture (TA, N = 631), electro-acupuncture (EA, N = 69) and sham acupuncture (SA, N = 423). The mean age of the participants ranged from 50.4 to 61 years with a median age of 54 years. The mean frequency of hot flashes at baseline ranged from 1.9 to 12.9 times per day with a median frequency of 8.4 times per day. The acupuncture frequency ranged from 0.7 to 12 times per week with a median frequency of 1.3 times per week. The treatment duration ranged from 4 to 24 weeks with a median time of 8 weeks. The sample size of each treatment arm ranged from 10 to 170 with a median value of 24 (Table 1).
|
Overall |
Traditional acupuncture (TA) |
Electro-acupuncture (EA) |
Sham acupuncture (SA) |
|
|---|---|---|---|---|
|
Number of arms |
30 |
13 |
4 |
13 |
|
Total sample size |
1123 |
631 |
69 |
423 |
|
Sample size per arm |
24(10–170) |
27(10–170) |
17(12–23) |
24(10–164) |
|
Treatment duration, week |
8(4–24) |
8(4–24) |
12(8–12) |
8(4–24) |
|
Age, years |
54.2(50.4–61) |
54.7(50.4–61) |
53.7(51.2–61) |
54.3(53-56.5) |
|
Mean frequency of hot flashes at baseline, per day |
8.5(1.9–12.9) |
8.7(1.9–12.9) |
8.0(6.3–9.6) |
8.1(2.2–12.3) |
|
Type of subjects, menopause/breast cancer |
19/11 |
8/5 |
2/2 |
9/4 |
Detailed information regarding the included studies is listed in Supplementary Table S1. Of the 17 studies, 3 (17.6%) were high quality, 14 (82.4%) were medium quality, and no studies were low quality. The quality of the 17 studies is shown in Supplementary Figure S2.
Model establishment and assessment
The time course of changes in hot flash frequency from baseline was well described by the Emax model. The goodness-of-fit plots of the acupuncture response model indicated a relatively good fit to the observed data (Supplementary Figures S3 and S4). Specifically, plots of population prediction (PRED) vs. observation (OBS) and plots of individual prediction (IPRED) vs. OBS were symmetrically distributed and close to the identity line, indicating good predictions. The plots of time vs. conditional weighted residual errors (CWRES) and plots of PRED vs. CWRES showed no trend and were randomly scattered around the identity line at CWRES = 0, indicating the suitability of the error model for this study. The VPC plots indicated that the 95% CI of model prediction covered almost all of the observed data, demonstrating good predictability by the model (Fig. 2). The results of LOOCV showed that the distribution of model parameters was stable and only slightly affected by individual trials (Supplementary Figure S5).
The covariate screening process revealed that the baseline frequency of hot flashes had a significant impact on the Emax value. The final model was expressed as follows:
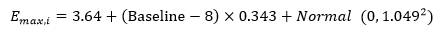 Equation 1
Equation 1
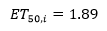 Equation 2
Equation 2
In Eq. 1, Emax,i was the Emax value for the ith group with 3.64 being the typical Emax value for the overall group and Baselinei was the frequency of hot flashes for the ith group at baseline. For every increase in the number of hot flashes at baseline, the Emax value increased 0.343 times. Therefore, the correction coefficient of the baseline for Emax was 0.343. Normal (0, 1.0492) was the inter-group variation of the Emax value using normal distribution with a mean of 0 and variance of 1.049. The optimal Emax,i values and the standard errors for each group as estimated by Bayesian feedback are shown in Supplementary Table S2. During the model building process, the inter-group variability of ET50 was close to zero, which means that the ET50 value of all the groups was close to 1.89 weeks. Finally, the inter-group variability of ET50 was fixed to 0 to enhance model stability in the final model (Eq. 2).
Typical efficacy analysis
As the frequency of hot flashes at baseline had a significant impact on the Emax value, it was necessary to perform baseline correction of the Emax value for each treatment group. The correction equation used was as follows:
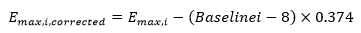 Equation 3
Equation 3
In Eq. 3, the Emax,i,corrected was the Emax correction value for the ith group and Emax,i was the Emax value for the ith group. This equation corrected the Emax values at different baselines to a level of 8, thereby eliminating the impact of the baseline frequency of hot flashes on Emax values when comparing the efficacy characteristics of the different treatment groups.
To compare the efficacy characteristics of different interventions, we summarized the Emax correction value for each group according to the intervention and estimated the 95% CI of the efficacy at week 8 for each type of intervention. The results revealed that the corrected Emax values of TA&EA and SA were 4.0 (95% CI: 3.6, 4.3) and 3.2 (95% CI: 2.7, 3.7), respectively. Further division of acupuncture into EA and TA resulted in corrected Emax values of 4.4 (95% CI: 3.9, 4.9) and 3.8 (95% CI: 3.5, 4.2), respectively (Table 2).
|
Intervention |
Arms (sample size) |
Emax (95% CI) |
Emax,corrected (95% CI) |
ET50 (95% CI) |
Corrected efficacy at week 8 (95% CI) |
|---|---|---|---|---|---|
|
TA |
13 (631) |
3.8(3.3,4.4) |
3.8(3.5,4.2) |
1.9(1.8,2.0) |
3.1(2.8,3.4) |
|
EA |
4 (69) |
4.4(3.5,5.3) |
4.4(3.9,4.9) |
1.9(1.8,2.0) |
3.6(3.2,4.0) |
|
TA&EA |
17(700) |
4.0(3.5,4.4) |
4.0(3.6,4.3) |
1.9(1.8,2.0) |
3.2(2.9,3.5) |
|
SA |
13 (423) |
3.1(2.3,3.9) |
3.2(2.7,3.7) |
1.9(1.8,2.0) |
2.6(2.2,3.0) |
|
Placebo pill△ |
11(2191) |
2.7(2.1,3.3) |
2.7(2.1,3.3) |
1.2(0.8,1.6) |
2.3(1.8,2.9) |
| TA: traditional acupuncture, EA: electro-acupuncture, TA&EA: merger analysis of traditional acupuncture and electro-acupuncture, SA: sham acupuncture. | |||||
| △The data of placebo pill was cited from Li, T. 2018 [27]. In this literature, the efficacy of placebo pill was not associated with the baseline frequency of hot flashes, thus the corrected Emax value of placebo pill was consistent with the original value. | |||||
Based on the corrected pharmacodynamic parameters described above, the efficacy of each intervention could be estimated at different time points (Fig. 3). For example, the efficacy at week 8 of treatment for TA&EA and SA were 3.2 (95% CI: 2.9, 3.5) and 2.6 (95% CI: 2.2, 3.0), respectively, and the efficacy of EA and TA were 3.6 (95% CI: 3.2, 4.0) and 3.1 (95% CI: 2.8, 3.4), respectively (Fig. 4).
Comparison of efficacy with drug treatment
Our previous studies showed that the efficacy of progesterone analogs, SSRIs/SNRIs, neuroleptic agents, tibolone, phytoestrogen, and placebo at week 8 of treatment were 6.4 (95% CI: 5.2, 7.7), 4.0 (95% CI: 3.5, 4.6), 3.5 (95% CI: 2.8, 4.2), 3.1 (95% CI: 2.6, 3.6), 2.6 (95% CI: 1.9, 3.4), and 2.3 (95% CI: 1.8, 2.9), respectively, after the baseline frequency of hot flashes was corrected to eight times per day. These results suggested that the efficacy of EA was comparable to SSRIs/SNRIs and neuroleptic agents, whereas TA was comparable to tibolone. SA trended to be slightly more effective than placebo pills, but the difference between them failed to reach statistical significance.