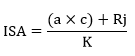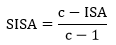Demonstration of the monthly changes in the population abundance of mosquito species, along with meteorological parameters is crucial in understanding the biology of potential vector species for efficient mosquito control strategies. This is the first comprehensive study on this topic in Mazandaran Province, northern Iran using the ISA/SISA indices in the expression of the most abundant species.
Vector abundance is a key determining factor that is often used as a risk indicator of vector-borne diseases. It is quantitatively more important than vector competence (although competence is a transmission requirement). It could explain the importance of abundant species in an area and why some vectors could contribute to epidemics without considering the competence for transmission [39, 40]. Culex pipiens is the most abundant species both in the larval and adult stages based on index of SISA/ISA in the present study. It was noted to be a common species in other studies in different parts of Iran [34, 41–44], but it should be mentioned that none of these researchers reported abundance of species based on the SISA/ISA index.
Although Culex pipiens was collected most frequently by total catch in animal places, it was the predominant species (based on index of SISA/ISA) in total catch in human places and shelter pit compared to other species, respectively. It indicates that the species tends to be attracted to humans, animals and also shows some extent of exophily in the study area. It is possible that humans come into contact with pathogens this species may carry. Such studies could establish baseline data for public health interventions in control programs, therefore, an assessment of different trapping methods is also required to adjust the design of future entomological and pathogen surveillance efforts [45]. The monthly activity of Cx. pipiens (larvae and adult) begins in mid-spring after diapause termination and reaches its largest peak in July and then declines again with an irregular fluctuations until the species disappears. It seems that the spring rains is a cause for the population of the species to peak in July, while no significant association was observed between Cx. pipiens and meteorological factors, probably high compatibility and access to diverse breeding places could help increasing population density of the species in early warm season. In contrast, Cx. pipiens was active from May to November and June to September [28, 46]. The population density of this species increased in July [46] and May to August [47], reaching its largest peak in August [46, 47]. Culex pipiens showed an increase in population density from May to July in north-eastern Croatia, then sharp decline occurred toward the end of the season [48]. In northern Italy, the species had maximum activity peak in July [49], that is almost consistent with the results of the present study.
There is not much data about the seasonal activity of culicine mosquitoes, especially in adult stage, in Iran [29]. The highest activity peak of Cx. pipiens was documented in mid-July in Guilan Province. There is no significant correlation between species population density and the meteorological data [29], which is in accordance with our research. Variations in the seasonal patterns of the species in this study and findings in other regions probably can be due to differences in the topography and climates.
Culex pipiens with its ornithophilic behavior and opportunistic feeder that bites both humans and animals, can be as bridge vector between birds and humans so is believed to be the principal vector of West Nile Virus [50]. It also shows an important role in transmission of several human pathogens including St. Louis encephalitis virus (SLEV), filarial worms as well as wildlife pathogens such as avian malaria [51]. The existence of swamps for migrating birds and their active presence throughout the Autumn and Winter in the province, detection of virus in the species in Guilan Province [52], can cause a concern for entry and the spread of the virus in the region.
Culex tritaeniorhynchus, the second most abundant species based on index of SISA/ISA in the study area, was first collected in May (larval stage) and June (adult stage), increased sharply in July, becoming the most abundant species, and displayed a rapid decline in December. The abundant species shifted from Cx. tritaeniorhynchus to Cx. pipiens in November, probably it could be due to high adaptability of Cx. pipiens to the environmental conditions in the area. In contrast, this species showed the highest peak of activity in August in Belek Region of Turkey [28]. While the peak of activity was observed in July in Guilan Province [29], which is in agreement with our study.
Culex tritaeniorhynchus is caught with the highest number by total catch in animal places which reflects the zoophilic tendencies of the species in the study area. Based on the results of a study in India, it is considered to be predominantly exophilic and normally zoophilic [53]. It was recorded to be the most abundant species in numerous studies in Iran, without reporting any SISA/ISA index [32, 33, 43, 54].
Culex tritaeniorhynchus is the primary vector of Japanese encephalitis (JE) in southern Asia. It has also been observed infected with dengue, Rift Valley fever, Sindbis, Getah and Tembusu viruses, and microfilariae of both Brugia malayi and Wuchereria bancrofti, in many areas of eastern and southeastern Asia [55]. The species is a principle vector of Rift Valley fever in Saudi Arabia [56], WNV in Asia [9] and a possible vector of Japanese encephalitis in Iran and Iraq [57], which indicates a high risk species for human health.
Among the Anopheles, An. maculipennis s.l. is numerically the most abundant species in the present study, mostly sampled by total catch methods in animal places, displayed to be predominantly zoophilic. This is consistent with other studies, but there is no evidence of SISA/ISA indexes to express the most abundant species in these studies. [42, 58–60]. What is interesting is that, despite having the highest numerical abundance of species, An. pseudopictus was calculated as the most abundant species in all sampling methods (except daily bites) based on ISA/SISA index. In addition, An. sacharovi also had the highest numerical abundance in the Shelter Pit, but it was not considered as the most abundant species based on the index of SISA/ISA.
Moreover, An. sacharovi had also the most abundant species in the shelter pit, but, it with regards to its high numerical abundance. Therefore, this shows the importance of species distribution at different sites and computational value of the index ISA/SISA Anopheles maculipennis s.l. is known to be the most important malaria vector in northern and western parts and the central plateau of Iran [61]. Moreover, there is a belief that this species can play a vector role in WNV transmission in various countries of the old world [62, 63]. Recently, the myxoma virus genome was detected in wild caught An. maculipennis that fed on wild rabbits by polymerase chain reaction (PCR) in Southern England, UK. Batai virus (BATV) and Anopheles associated C virus (AACV) was also identified and isolated from Anopheles maculipennis complex in entomological surveys in Germany, Italy and France [64]. With regards to the malaria historical records and WNV in the northern parts of Iran [52, 65], understanding the population dynamics and the maximum monthly peaks of the species is important in controlling the diseases in the study area.
The highest monthly activity peak of An. maculipennis (larvae and adult) is in June. In contrary to our results, An. maculipennis s.l. showed the highest peak of activity in the mid-July in Guilan [29] and July–August in Kalaleh County of Golestan Province [30], northern Iran. In neighboring Turkey, the species peaked in August and July–August [66, 67]. These differences in population patterns could possibly be due to regional ecological differences. On the other hand, some researchers believe that these discrepancy might also be due to the low collection effort involved in the studies [26] or sampling regimes [28, 29, 49, 66, 67].
Climate change and the environment affect the abundance and distribution of vectors and their intermediate hosts [68]. In the present study, monthly population fluctuations of Cs. annulata, Cs. longiareolata and Ae. vexans are correlated with rainfall. Although these species showed irregular monthly population fluctuations, they were more active in spring and autumn. Culiseta annulata had a major peak in November [28], which is in accordance with our research. On the other hand, Cs. longiareolata was active in July and September [66], whereas in the present study, the species had the largest peak in October, which is quite similar to that of Ae. vexans.
The largest peak of Ae. vexans was reported in June [66] and August [67].There are evidence that Ae. vexans and Cs. annulata are important vectors of WNV [69, 70]. Among the Aidini tribe, Aedes vexans is recorded to be the most abundant species based on ISA/SISA index. It was caught in all traps and is the most abundant collected species in day biting compared with other species, and shows anthropophilic tendencies in the study areas. This species demonstrated high prevalence and collected by day biting in other studies performed in different parts of Iran [58, 71–73], this may be a reflection of the fact that this method has a high level of efficiency for collecting this species. However, to observe ethical values, this collection methods cannot be used freely and with extended time and should in fact be restricted or modified in compliance with ethical standards [74].
The species is known to be the main vector of Tahyna virus in central Europe [75], potential vector of the dog heartworm Dirofilaria immitis in Europe [76] and principal vectors of Rift Valley fever in Saudi Arabia [56]. Recently, Zika virus is revealed in the salivary glands of the field-caught Ae. vexans [77]. It is a flood water mosquitoes, so, their population abundance depends on the water dynamics of temporary pools [26]. Rainfall as an important climatic factor may have a range of different correlation with the population of the species, from positive, negative and/or ineffective and sometimes with a lag phase [78] a significant positive correlation between its monthly activity and rainfall was observed in our study. The current study was not designed and aimed to address the analysis of lag time between climate factors and population density of the species, however, it seems that there is probably a lag time of at least 15 days after rain at the beginning of the rainy season before the Ae. vexans population jump start. In concordant with our findings, there is a lag time of at least 15 days between the peak of rainfall and abundance of the species [79–81]. Moreover, other studies have revealed a correlation between rainfall and the abundance of species with a 10-day lag in the early rainy season and 20 days after the end of the rainy season [82]. In the study of Diallo et al. [81] rainfall patterns displayed that heavy rains between August and September 2005 had negatively impacted on the population density of Ae. vexans, whereas in 2006, population peak was observed following the rainfall peak. These findings show the complexity of the relationship between climate factors and population density of mosquitoes. Therefore, it is recommended to measure the seasonal activities of species over multiple years, to show a better understanding of the correlation between population frequency and climatic factors, and the impact of other variables.
The highest number and population density of Ae. vexans was found during early autumn, especially in October. Vector-borne diseases show seasonal patterns with inter and intra-annual variability, which are partly described by climate and environmental factors [79]. Therefore, these results are important for health authorities in controlling mosquitoes as well as in the tourism industry for nuisance control, but more importantly, it provides a detailed estimate of the timing of risk for human populations.