Methods
Study Design and Patients: A randomized double-blind placebo-controlled trial was conducted involving 18 athletes and 18 healthy man volunteers. The inclusion criteria were: 1) Athlete volunteers who have national sports rank or players of the professional soccer or any sports leagues; 2) At the age range of 20 to 30 years old; 3) BMI between 18.5 to 25 kg/m2; 4) Intake of omega-3 less than 1600 mg/day according to food frequency questionnaire (FFQ: 147 items) in last year; 5) Avoidance of any weight reducing drugs, dietary supplements, vitamins, minerals, and protein powders at least 6 months before and throughout the intervention; 6) Not having coagulopathy blood disease, kidney disease, liver damage, pancreatitis, diabetes, cancer, thyroid disorders, and inflammatory diseases or history of heart disease and stroke according to the individual statement; 7) No smoking; 8) Normal blood lipid profile tests before the start of the study. The exclusion criteria were: 1) Allergic reaction to the compositions of omega-3; 2) Unwillingness for cooperation; 3) Any drastic change in diet, duration, level, or type of physical activity and regular lifestyle; 4) Consuming alcohol, and smoking.
Following a public announcement of the study, volunteers willing to participate were recruited from public and private gyms, teams, stadiums, councils, and departments of sports and youth, department of physical education, sports medicine board in the Tabriz, Iran. The participants were the athletes in at last one filed (football 9, volleyball 3, basketball 4, athletics 2, archery 1, martial arts 6, swimming 4, weightlifting 3, wrestling 1, rock climbing 3). Participants who meet the eligibility criteria and be agree to enroll to study, after being given a full explanation of the study procedures, signed a statement of informed consent was taken from each participant before the commencement of baseline data collection. The present study was conducted according to the guidelines of the Helsinki Declaration. The study was approved by the Ethics Committee of the Tabriz University Medical of Sciences (IR.TBZMED.REC.1398.782) in October 2019, and the trial was registered at the Iranian registry of the clinical trial website (www.irct.ir) as IRCT20190625044008N1 (https://en.irct.ir/trial/43332).
Randomize, Blinding, and study method: For randomization, a blinded colleague who was not involved in any of the study stages randomly divided the participants into the intervention and placebo groups (1:1) by using RAS (Random Allocation Software). Allocation concealment was achieved through the use of closed letters with consecutive numbering. The blinded assistant created a 2-digit code for each participant to facilitate identification and tracking. Blinding of participants and investigators was supported through the provision of omega-3 or placebo containers with identical labeling. Placebo and supplements must be similar in terms of color, shape, and size. Gelatin capsule supplements and placebo will be stored at room temperature. Therefore, subjects and investigators will not be known for the treatment assignments in the study. Participants and researchers were all blinded to participant group members throughout the study. Subjects assigned to the omega-3 (n=18) and placebo (n=18) groups, using a randomized block procedure with stratified subjects in each block based on metabolic equivalents (METs) from GPAC physical activity questionnaire. So participants will be classified into 4 groups: 1) the omega-3 group with high MET receiving supplements of two omega-3 soft gel capsules per day, Zahravi Pharmaceutical Co, Tabriz, Iran, consists of 240 mg of DHA, 360 mg EPA.
2) The omega-3 group with low and moderate MET receiving supplements of omega-3.
3) The placebo group with high MET receiving placebo two soft gel capsules per day, each capsule containing one g of edible paraffin oil; provided by Zahravi Pharmaceutical, Co., Tabriz, Iran.
4) The placebo group with low and moderate MET receiving paraffin soft gel capsules.
Intervention: All the participants received supplements or a placebo for three weeks. All participants were asked to take the capsule with their main meal. To keep informed of the regular intake of supplements and possible problems during the study, we called with participants once every week and also on the second visit asked them about how to consume supplements. Participants were asked to return boxes of drugs and the compliance of participants was evaluated by counting the number of unconsumed capsules at the end of the course and the subjects with less than 90% consumption were excluded. (None of the participants who complete the trial, had compliance less than 90%). Every week compliance of the sports activity and diet, evaluated by phone as a question of whether the diet or physical activity has changed a lot or not. (None of the participants excluded because of this reason).
Assessment of Physical Activity and Dietary Intake: The global physical activity questionnaire (GPAQ) was used to estimate physical activity levels. A trained researcher filled out the questionnaire for each participant before and after the intervention, via face-to-face interview [34]. The validity and reliability of GPAQ have been confirmed previously by BashiriMoosavi et al. [35]. Data processed according to guidelines for analysis of the GPAQ that a total metabolic equivalents score (MET-minutes/week) was calculated, with patients categorized as high (≥3000 MET), moderate (600–3000 MET), or low (<600 MET) levels of activity (just high and low METs included the intervention). Changes in Dietary intake was assessed using a 24-hour recall questionnaire at the beginning, the same days every week, and at the end of the study is taken to ensure dietary intake didn’t significantly change. FFQ was completed before the intervention, by a trained nutritionist, to assess the omega-3 intake in the past year. Nutritionist IV software (First Databank, San Bruno, CA, USA), modified for Iranian foods, was used for dietary data analysis.
Blood Sampling and Biochemical Measurements: 10 to 12 hours night fasting blood samples (5 mL) in sterile tubes with EDTA as an anticoagulant (Vacutainer K2E) was drawn for biochemical analyses. For serum separation samples are centrifuged at 3000 RPM for 5 minutes then 3 ml of whole blood samples are collected in a sterile microtube without any anticoagulant and store at -80 ° C until the serum markers of the study measured. Blood profile indexes including Serum Total cholesterol (TC), triglycerides (TG), and high-density lipoprotein (HDL-C) were measured through enzymatic methods using the colorimetric technique, by commercial kits (Pars-Azmoon Co., Tehran, Iran). LDL is calculated by the Friedewald equation[36].
Anthropometric measurement: Anthropometric parameters including weight and height were measured. The weight of the participants measured with minimal clothing and without shoes by digital scale to the nearest 0.05 g{Organization, 1995 #1095}. Height measured using a wall stadiometer in standing position without shoes with a precision of 0.5 cm (Seca, Germany) [37]. Wrist circumference measured using a tape meter to the nearest 0.1 cm. BMI was calculated as weight (kg) divided by height squared (m2). Body composition evaluated using Tanita MC-780 S MA (Amsterdam, the Netherlands). RMR was measured by indirect calorimetry using Fitmate Pro (Rome, Italy). The measurements were performed by a trained nutritionist. Besides, blood pressure was measured in a comfortable sitting position in the left arm after at least five-minutes resting, using an aneroid sphygmomanometer and stethoscope. It was measured on two occasions and the mean of the two was taken as the individual’s blood pressure.
Sample Size and Statistical Analysis: Considering the differences between the two studied groups for one of the main outcomes, we calculated the sample size as follows:
Z1-α/2=1.96 α= 0.05 1-β=0.90 Z1-β= 1.282
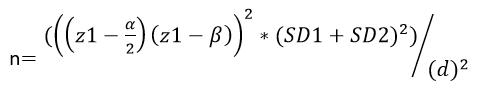
According to the equation above, the sample size was calculated by nearly 14 in each group and we selected 18 in each group, including a possible 30% loss to follow-up and discontinued intervention, and for great accuracy.
Statistical analysis was done by STATA software [ver.16] (StataCorp, College Station, Texas 77845 USA). Normality of the numeric variables checked by Kolmogorov- Smirnov test. Data expressed using mean (SD), median (min-max) for the numeric normal and non-normal variables respectively, and frequency (percent) for categorical variables. The between-group comparisons of baseline measures and demographic variables done by independent t-test, Mann-Whitney U test, and/or Chi-square test where appropriate. For within-group comparisons paired t-test and Wilcoxon sign-rank test used for the numeric normal and non-normal variables, respectively, before and after intervention measurements were taken. To assess the effect of intervention the analysis of covariance (ANCOVA) used controlling for baseline measures and confounders. In all analyses, P values less than 0.05 considered as significant.